CHAPTER 41 Pediatrics
Most veterinarians have been presented with kittens that have failed to thrive, often called “fading kitten syndrome.” These patients are challenging due to their small size, their unfamiliar physiology, and the tendency for their status to deteriorate quickly. Sick neonates should be examined as soon as possible with a systematic approach, including a complete history of the kitten, litter, and queen; examination of the kitten and queen; and diagnostic tests.21,52
Kitten Morbidity and Mortality
High-risk time points for kitten morbidity and mortality are at birth, in the first 2 weeks of life, and around the time of weaning. There is no specific disease entity attributable to “fading kittens,” but rather a variety of causes have been identified. “Toxic milk syndrome” is often implicated when the cause of neonatal illness or death cannot be determined. There is no evidence that such a syndrome exists in kittens, and attention should be focused on finding the actual cause of illness or death. The most common causes of morbidity and mortality in kittens include20,39
• Perinatal events (e.g., dystocia, poor maternal care)
• Failure of passive transfer of immunity
• Environmental factors (e.g., poor ventilation, temperature fluctuations, overcrowding)
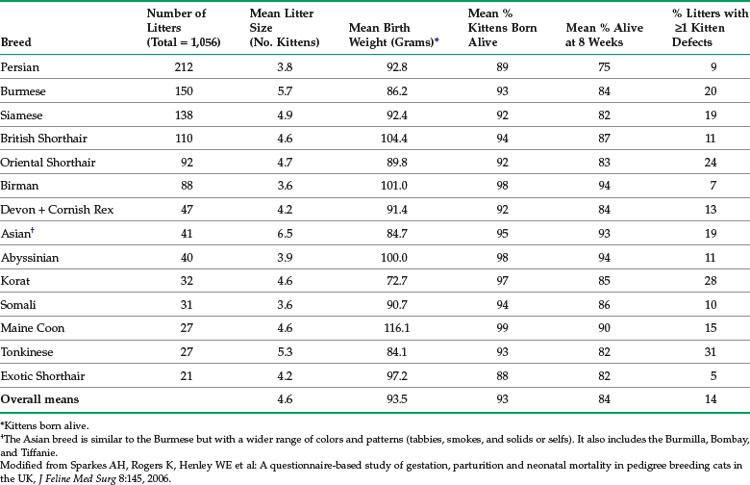
In free-roaming populations, kitten mortality may be as high as 75%, with trauma and infectious disease accounting for most deaths.60 The lowest mortality rates (less than 5%) are found in well-managed, specific–pathogen-free colonies. Pedigreed breeding catteries are a unique opportunity to collect feline reproduction data, including data on neonatal health and illness. In a study in the United Kingdom, data were collected using a convenience-sampling questionnaire on the births of 1056 litters (4814 kittens), representing 14 pedigreed breeds.75 The breeds included in the study were Persian, Burmese, Siamese, British Shorthair, Oriental Shorthair, Birman, Devon and Cornish Rex (data analyzed together), Asian, Abyssinian, Korat, Somali, Maine Coon, Tonkinese, and Exotic Shorthair. Several parameters were affected by breed, such as mean stillbirth rate (highest in Persians and Exotic Shorthairs, lowest in Maine Coon) and mean kitten birth weight (highest in Maine Coon, lowest in Korat). Selected data from this study are summarized in Table 41-1.
TABLE 41-1 Breed-Specific Reproduction Data Collected Using a Questionnaire-Based Survey in the United Kingdom
In another study of 1191 litters (4804 kittens), data were collected prospectively using an Internet-based convenience-sampling submission system on 11 breeds (S. Little, unpublished data, 2001 to 2005). Breeders submitting data were located primarily in the United States and Europe. The breeds included in the study were Bengal, Birman, Egyptian Mau, Havana Brown, Manx, Munchkin, Norwegian Forest Cat, Ocicat, Ragdoll, Devon Rex, and Sphynx. The mean stillbirth rate was highest in the Havana Brown and lowest in the Ocicat and Devon Rex. Mean kitten birth weight was highest in the Norwegian Forest Cat and lowest in the Havana Brown. Selected data from this study are summarized in Tables 41-2 and 41-3.
TABLE 41-2 Breed-Specific Reproduction Data Collected Using an Internet-Based Survey from Breeders Primarily in the United States and Europe, 2001 to 2005
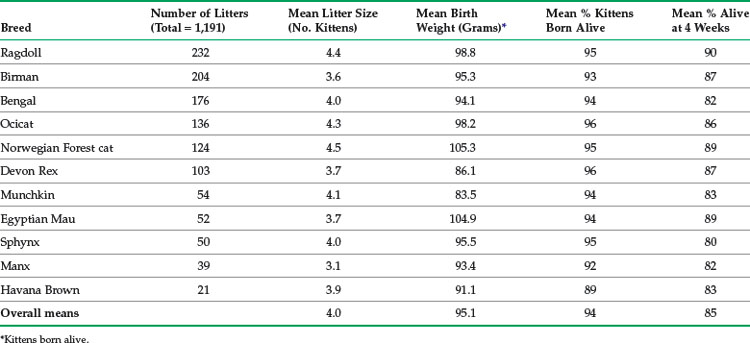
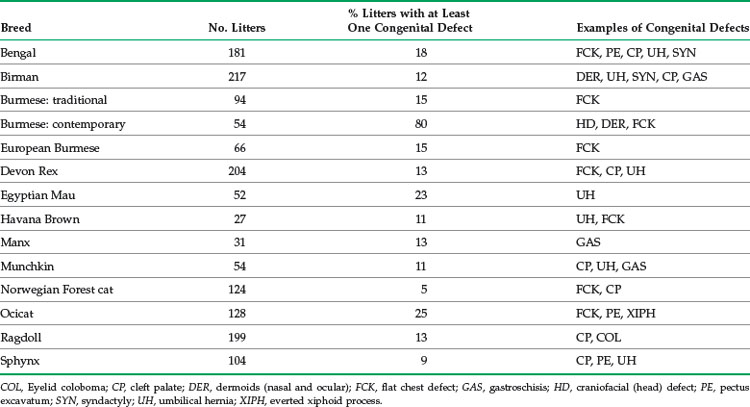
TABLE 41-3 Data on Congenital Defects in Selected Pedigreed Cat Breeds Collected Using an Internet-Based Survey from Breeders Primarily in the United States and Europe, 2001 to 2005
Data from such studies are very useful to help breeders evaluate their breeding programs and identify areas of poor performance. For example, mean kitten mortality by 4 to 8 weeks of age in these studies is approximately 15%. A study in Sweden involving 694 litters from various breeds calculated mean kitten mortality by 12 weeks of age as 18%.76 Breeders experiencing greater losses should investigate possible causes. Caution must be exercised, however, because even though breeds may have the same name and similar phenotype in different countries, the genetic constitution may be quite different. A good example is the Burmese breed. The definition of this breed varies by country and registering organization, leading to genetically distinct populations with different disease risks.
Examination of the Neonatal Kitten
Investigate the kitten’s home environment, noting temperature and humidity, sanitation, population size and density, and prevalence of infectious diseases and parasites. A home visit can gather important information, especially when working with breeders. However, if a home visit is not possible, the breeder can be asked to supply a floor plan and photos to help identify management issues. Unfortunately, little information exists on the optimum design of breeding catteries. Recommendations are often adapted from those designed for laboratory animals, or boarding facilities.66 The Cat Fanciers’ Association (http://www.cfa.org) has produced minimum requirements for catteries that address the environment (e.g., temperature, ventilation, lighting), hygiene, and caging facilities. Although written about 20 years ago, valuable information on cattery design is found in Feline Husbandry: Diseases and Management in the Multiple-Cat Environment (http://www.vetmed.ucdavis.edu/ccah/felinehusbandry.cfm).62
One of the first challenges facing the clinician examining the neonatal kitten is to determine the age and sex. Unless the kitten comes from a breeding cattery, the exact birth date is often unknown. Several developmental milestones and parameters can be used to estimate the age of kittens (Box 41-1), such as dentition and body weight (see below). Sex may be surprisingly difficult to determine in very young kittens, especially without another kitten of opposite sex for comparison, because testicles are not readily visible until more than 6 weeks of age. In male kittens, the distance between the anus and genitals is greater (about 1.25 cm [0.5 inches]) than for female kittens. The genitals appear slitlike in female and appear rounded in the male. Coat color may also be a clue; almost all calico or tortoiseshell kittens are females, and orange kittens are most likely to be male (but not exclusively so).
BOX 41-1 Developmental Milestones for Kittens
Umbilical cord falls off: 3 days of age
Eyelids open: 10 days of age (range 2 to 16 days)
Menace/pupillary light reflexes: 28 days of age or later
Adult iris color: 4 to 6 weeks of age
Ear canals open: 9 days of age (range 6 to 17 days)
Functional hearing: 4 to 6 weeks of age
Voluntary elimination: 3 weeks of age
Deciduous incisors/canines erupt: 3 to 4 weeks of age
The typical kitten birth weight is 90 to 110 g (range, 80 to 140 g), although there is considerable variation by breed (see Tables 41-1 and 41-2) and by sex (males typically weigh more than females).75 Low birth weight is a common cause of mortality, with kittens weighing less than 75 g at birth at highest risk. Slight weight loss (less than 10%) can occur in the first 24 hours of life, but the kitten should then gain weight daily. Normal kittens gain 50 to 100 g per week (10 to 15 g/day) and should double their birth weight by 2 weeks of age. Breeders and other caretakers of newborn kittens should be instructed to weigh the kittens twice daily for the first 2 weeks of life, and then daily for at least the next 2 to 4 weeks. Often the earliest sign of illness is failure to gain weight in a 24-hour period.
Inspect the kitten for gross anatomic abnormalities, such as cleft palate or lip, umbilical hernia or infection (omphalophlebitis), open fontanelles, limb deformities, chest wall deformities, and nonpatent urogenital or rectal openings. The normal umbilical cord is dry, with no redness, swelling, or discharge at the umbilicus (Figure 41-1). Umbilical cords will fall off at about 3 or 4 days of age.
Neonatal kittens may have lower blood pressure than adults, as well as greater cardiac output, and a faster heart rate in the first 2 weeks of life. Functional murmurs may be present in neonates because of anemia, hypoproteinemia, fever, or sepsis. Innocent murmurs not associated with disease are more common in puppies than kittens; murmurs still present after 4 months of age should be investigated. Congenital heart disease usually produces murmurs that are loud and accompanied by a precordial thrill. The normal neonatal heart rate can be more than 200 beats per minute in the first 2 weeks of life (range 220 to 260). By 4 weeks of age, once vagal tone has been established, the heart rate decreases to the normal adult range. The normal respiratory rate is 15 to 35 breaths per minute. See Box 41-2 for normal physiologic values for neonatal kittens.
BOX 41-2 Normal Physiologic Values for Neonatal Kittens
Rectal temperature (newborn): 97° F to 98° F (36° C to 37° C)
Rectal temperature (1 month): 100oF (38° C)
Heart rate: 220 to 260 beats/minute for the first 2 weeks of life
Respiratory rate (newborn): 10 to 18 breaths/minute
Respiratory rate (1 week): 15 to 35 breaths/minute
Urine specific gravity: <1.020
Water requirement: 130 to 220 mL/kg/day
Caloric requirement: 20 kcal ME/100 g/day
Stomach capacity: 4 to 5 mL/100 g
Congenital Defects
Congenital defects are abnormalities of structure, function, or metabolism that are present at birth. A defect may cause physical impairment, or it may cause the death of the kitten before or after birth. Congenital defects in stillborn kittens often go unrecognized, because few stillborns are submitted for complete necropsy. Many congenital defects are cosmetic or minor, while others may cause serious impairment of health (Box 41-3). Congenital defects may be of various types:
• Obvious at birth (e.g., cleft palate [Figure 41-2] or imperforate anus [Figure 41-3])
• Found only with diagnostic testing or at necropsy (e.g., diaphragmatic hernia)
• Subtle abnormalities found only with sophisticated testing (e.g., lysosomal storage diseases)
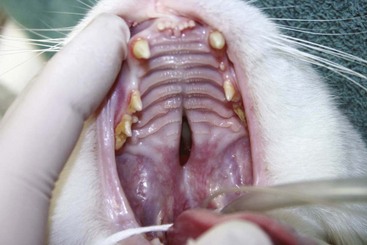
FIGURE 41-2 All kittens should be examined at birth for the presence of cleft palate, a common congenital defect.
(Courtesy Chris Carter.)
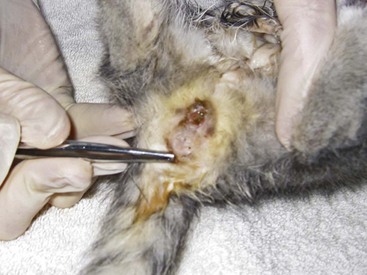
FIGURE 41-3 This female kitten has an imperforate anus and an anovaginal fistula.
(Courtesy Rosalyn MacDonald.)
Congenital defects may be heritable, and the inheritance pattern or mutation(s) responsible may or may not be known (see Chapter 44). A few congenital defects are due to chromosomal abnormalities, such as pseudohermaphroditism. Many congenital defects are not heritable but caused by other factors (Box 41-4). In some cases, defects may be caused by interplay of both environmental and genetic factors. When pedigreed breeders encounter a congenital defect where no information is available on heritability, several factors can be evaluated. A defect is more likely to be heritable if there is evidence of a breed or familial predisposition and the problem has a consistent age of onset and clinical course. A defect is less likely to be heritable if more than one abnormality occurs in a kitten or a litter or there is potential exposure to teratogens. An informative fact sheet (“What to do if your cat produces a deformed kitten?”) has been produced by the Feline Advisory Bureau to advise breeders on steps to take if a defect is observed (http://www.fabcats.org).
BOX 41-4 Examples of Nongenetic Causes of Congenital Defects in Kittens
As in many other species, congenital defects are a significant contributor to neonatal mortality in the cat. Excellent reviews of congenital defects have been published,31 including neurologic,38 ocular,25,56 renal,26 cardiac,45 and vertebral column defects.57 There are also studies of congenital defects specifically in pedigreed cat breeds in the literature. One study noted that congenital disease was more common in pedigreed kittens than in the nonpedigreed kittens in a necropsy examination of 274 kittens aged up to 16 weeks.9 However, the difference was not statistically significant and no individual breed of cat was significantly predisposed to congenital diseases in the data.
In a survey of 3468 pedigreed kittens in the 1970s, 6.8% had malformations.70 Individual breeds ranged from no defects reported to 17% (Colorpoint Shorthair) and 19% (Manx) of kittens affected. Another study from the same time period reported 4.2% of Burmese kittens from one cattery had congenital defects, as well as 12.7% of Persian kittens from four catteries.71 The types of abnormalities reported included heart defects, open fontanelles, gastroschisis, eye and eyelid defects, and gastrointestinal tract defects.
In the United Kingdom analysis of reproduction data from 14 breeds,75 14.9% of the litters included one or more kittens with congenital defects, ranging from 6% of Devon Rex litters to 31% of Tonkinese litters (see Table 41-1). The survey of cat breeders conducted primarily in North America and Europe (S. Little, unpublished data, 2001 to 2005) found wide variation in prevalence of congenital defects, from 5% (Norwegian Forest Cat) to 80% (“contemporary” American Burmese) of litters demonstrating at least one defect (see Table 41-3). The high prevalence of congenital defects in “contemporary”-style American Burmese is due to a common craniofacial deformity caused by an autosomal recessive genetic mutation.59 Congenital defects common to many breeds include thoracic wall deformities (e.g., pectus excavatum, flat chest defect), cleft palate, and umbilical hernia. Although certain common congenital defects of kittens are well described in the literature, others are less well documented.
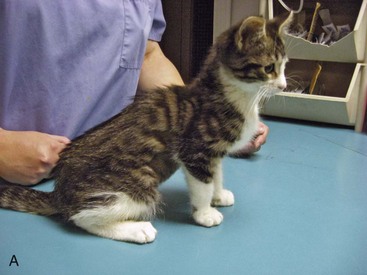
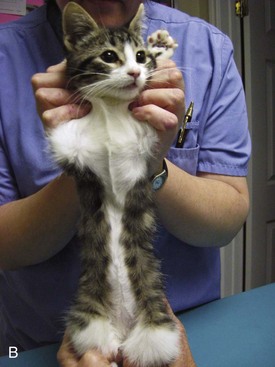
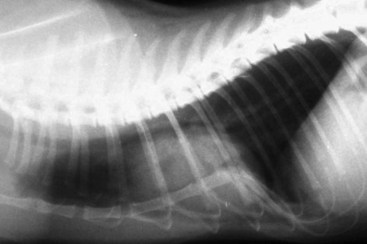
Flat chest defect is one of the common thoracic wall deformities in kittens that are not well described in the literature. Although the defect may be seen in any kitten, it appears to be most common in Orientals, Burmese, and Bengals. The defect is characterized by a dorsoventral flattening of the rib cage and sharp angulation at the costochondral junction (Figure 41-4).78 Curvature of the cranial thoracic spine may also be present (Figure 41-5). The defect is not present at birth, but the mean age at which it is recorded is 9.5 days.78 The defect has a variable presentation and is even transient in some cases. Mildly affected individuals may be difficult to detect. Moderate to severely affected kittens show poor weight gain, increased respiratory rate and effort, exercise intolerance, and they may die. In one study of Burmese kittens in the United Kingdom, 8.7% of the kittens with flat chest defect also had pectus excavatum, suggesting a possible association.78 Although similar thoracic wall deformities have been reported in taurine-deficient kittens, affected Burmese kittens had higher whole blood taurine levels than unaffected kittens.78 No investigation into the best treatment for kittens with life-threatening chest wall deformity has been published, but breeders and owners often apply temporary splints made of cardboard or plastic to force the compliant thoracic wall and sternum into a more normal confirmation until the ribs and sternum mature (Figure 41-6).

FIGURE 41-4 The flat chest defect is characterized by a dorsoventral flattening of the rib cage.
(Courtesy Barbara Hickmann.)
Pectus excavatum (“funnel chest”) is characterized by dorsal deviation of the caudal part of the sternum (Figure 41-7). The sternal abnormality is noticed early in life, and the defect is usually progressive. The etiology in kittens and puppies is poorly understood, although reviews of the disease and its management have been published.5,17 No breed or sex predisposition has been identified. The disease may be classified as mild, moderate, or severe based on radiographic measurements.17,18 Mildly affected kittens typically have no clinical signs and do not require intervention. Severely affected kittens may be dyspneic, exercise intolerant, and fail to thrive. A heart murmur may be present, either resulting from congenital disease or secondary to abnormal positioning of the heart because of the sternal deformity.18 The most commonly described surgical correction is percutaneous circumsternal sutures and external splinting (Figure 41-8).18,19,50,72,85 This technique works well in kittens with a nonossified sternum (less than 4 months of age), because the goal is to pull the sternum outward and maintain the correct position while the sternum matures. Potential complications of the technique include inadvertent lung puncture or laceration and development of pneumothorax. One case of fatal reexpansion pulmonary edema has also been described.72 Surgical approaches, such as wedge ostectomy,72 trans-sternebral pinning,13 and sternal wedge chondrectomy with internal splinting64 have also been described.
Tarsal hyperextension (also known as “twisted legs,” limb contracture, tendon contracture) is a recently described congenital defect in kittens.7 The defect is obvious at birth and typically affects only one kitten in a litter. There is no breed or sex predisposition. The abnormality is characterized by severe tarsal hyperextension and metatarsal rotation (Figure 41-9). Typically both hind limbs are affected, but the condition can also be unilateral. No bone abnormalities or neurologic deficits are present. The etiology is unknown, but the defect appears similar to clubfoot in human infants. In the author’s experience, the deformity completely resolves on its own in the majority of cases as the kitten begins to crawl and bear weight on the affected limbs. In some cases, delayed resolution has prompted the use of either soft molded splints or fiberglass casts for external coaptation.7 If external coaptation is required, it should be instituted earlier rather than later to take advantage of the greater flexibility of the joints of young kittens. Splints and casts must be changed weekly as the kitten grows, and may be required for 6 weeks or longer. Physical therapy to gently manipulate the tarsal joint into normal configuration may also be helpful.
Neonatal Diagnostics
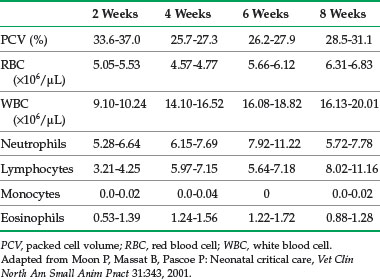
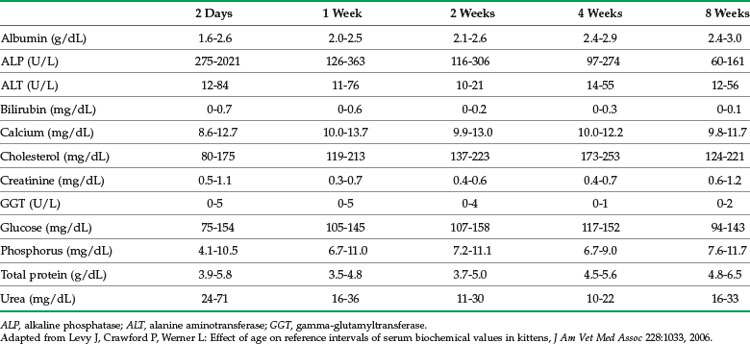
Blood chemistry and hematology values for neonates differ from the adult; most values normalize to adult levels by 3 to 4 months of age (Tables 41-4 and 41-5). For several analytes, reference ranges change rapidly within the first few days and weeks of life so that using age-appropriate reference ranges is important. In one study of kittens up to 8 weeks of age, reference ranges for alkaline phosphatase (ALP), creatine kinase, triglycerides, calcium, and phosphorus were higher than for adults.41 Reference ranges for aspartate aminotransferase (AST), bilirubin, urea nitrogen, and creatinine were higher in newborns, but similar to or lower than adults by 8 weeks of age. Reference ranges for albumin and total protein were lower than for adults for the entire 8 weeks, and values for calcium and phosphorus were higher.
For venipuncture, the holder positions the kitten in dorsal recumbency with the forelegs drawn back toward the abdomen and the head and neck extended. Blood is drawn from the jugular vein using a 1-mL syringe with a 25- or 26-gauge needle. Slow aspiration of blood is essential to avoid collapsing the vein. A small volume (0.5 mL) of blood can be used for the most critical tests (Box 41-5). Use of 0.5-mL microsample blood collection tubes is recommended and has been validated for evaluation of biochemistry and hematology samples.82,83 Repeated sampling should be done cautiously, because the circulating blood volume of kittens is small (approximately 70 to 95 mL/kg). Daily blood sampling should not exceed 10% of the kitten’s estimated total blood volume.
BOX 41-5 Minimum Database for Sick Neonatal Kittens
• PCV and total solids using microhematocrit tubes and refractometer
• Complete blood count: white blood cell count from one drop whole blood directly into Unopette, differential from blood smear
• Blood urea nitrogen with whole blood on reagent strip
• Blood glucose determined with drop of whole blood using glucometer (note these machines tend to underestimate blood glucose)
Urine is collected for chemistries, microscopic examination of sediment, and specific gravity by stimulating the perineum; cystocentesis should be performed with great care or avoided in the very young, because the bladder wall is easily lacerated. Urine specific gravity is 1.020 or less in the first few weeks of life; adult values are reached by about 8 weeks of age.46 A fecal sample should be examined for common intestinal parasites, such as Giardia spp., Isospora spp., and roundworms, using both zinc sulfate centrifugation and a direct saline smear.
Ultrasonography is an effective modality for pediatric patients, especially for imaging the abdomen. Machines with a curvilinear variable frequency scan head (6.0 to 8.0 MHz) have been recommended.3 Sedation is rarely required for the procedure, and the kitten is best positioned in dorsal recumbency in a padded trough. Techniques for examining the abdomen and the normal appearance of structures have been described.3 Common indications for abdominal ultrasonography include gastrointestinal foreign bodies, intussusceptions, congenital hernias, congenital renal disease, and urolithiasis, among others.
Normal electrocardiographic values for kittens in the first 30 days of life have been described.44 Changes in normal findings occur during the first month of life, such as shift of the electrical axis from right to left, a progressive increase in R wave amplitude, and a progressive decrease in S wave amplitude. Measurements for P wave, PR intervals, duration of QRS complexes, and the duration of the QT interval are similar to adult cats. Neonatal kittens have a sinus heart rhythm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 inch long. Developmental errors that occur during the first 2 weeks of gestation are usually lethal. It is also important to note that a defect in the development of one organ system or structure can result in the abnormal development of other organs or structures.
inch long. Developmental errors that occur during the first 2 weeks of gestation are usually lethal. It is also important to note that a defect in the development of one organ system or structure can result in the abnormal development of other organs or structures.