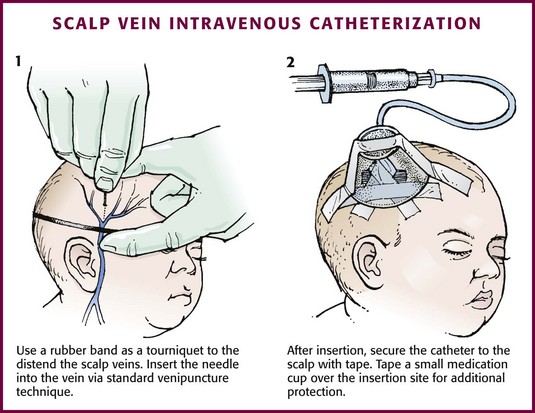
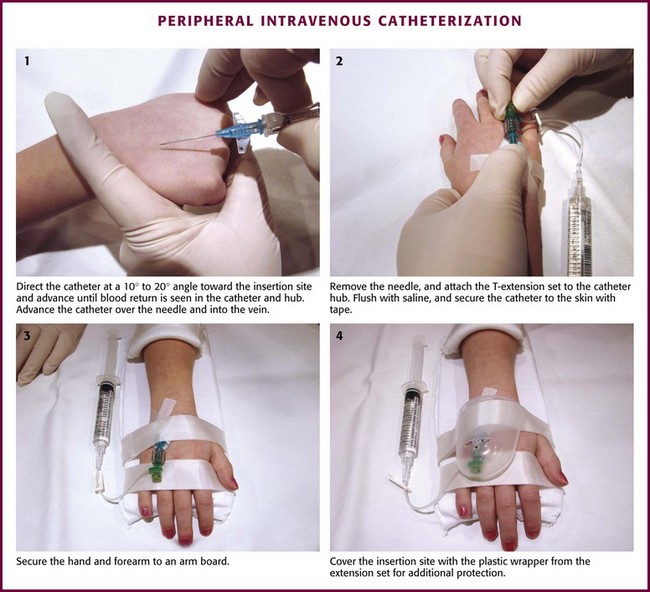
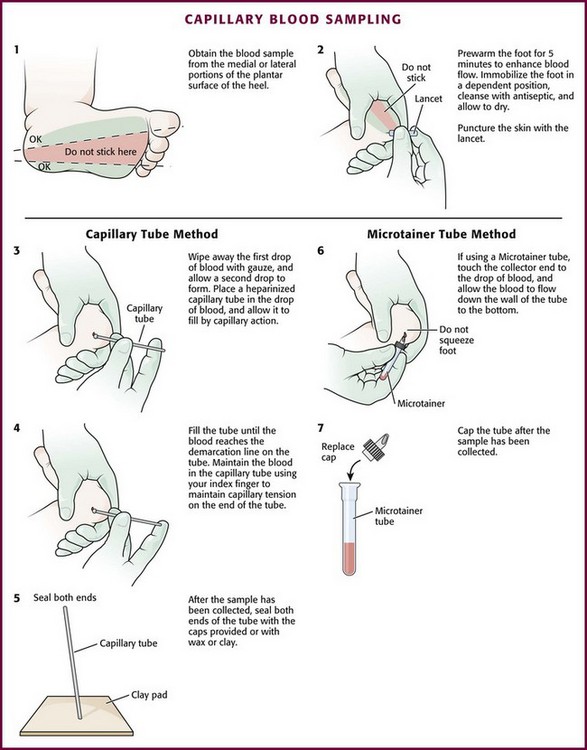
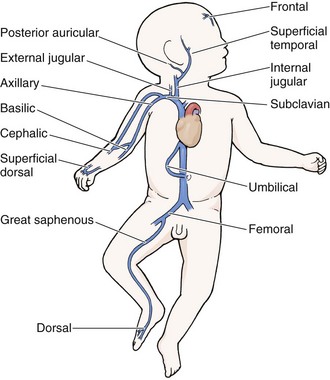
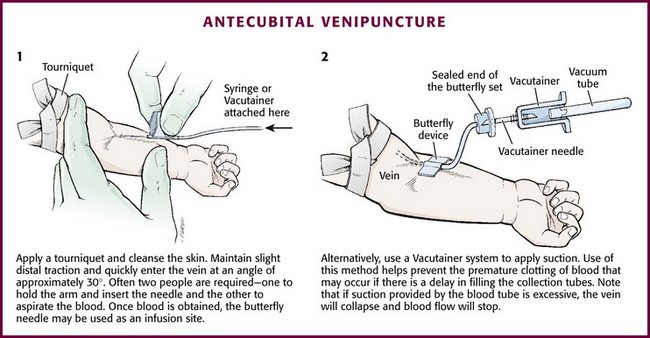
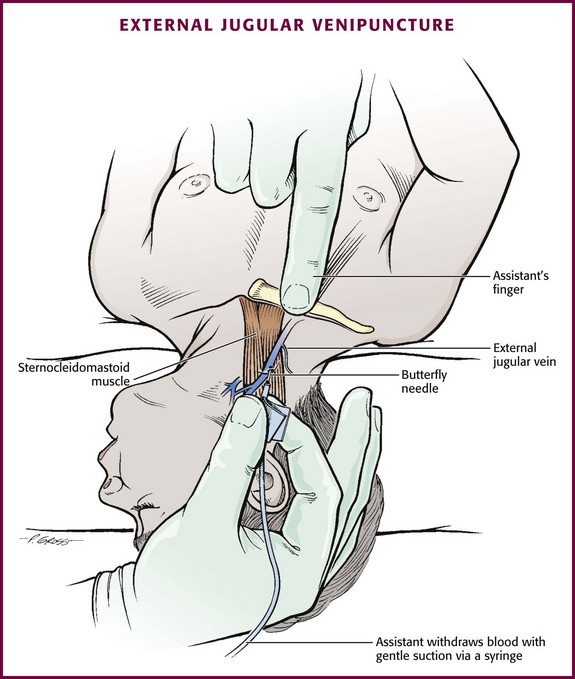
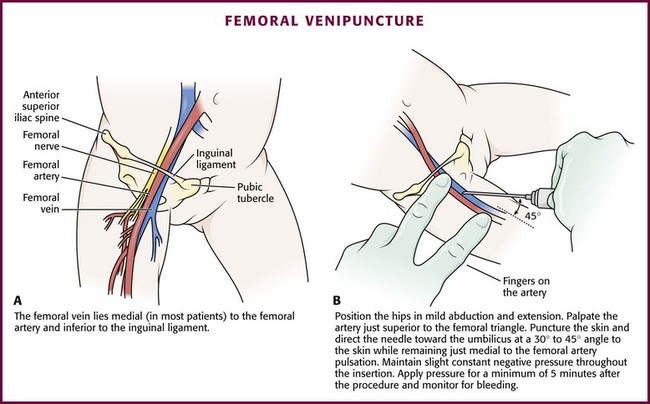
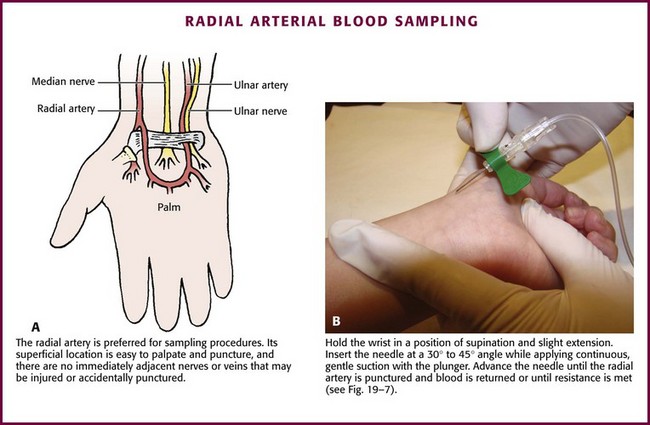
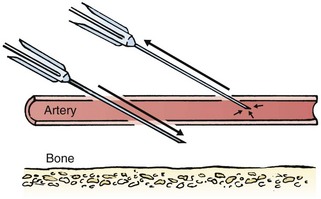
Chapter 19 Obtaining vascular access and blood samples in an infant or child can challenge and frustrate even the most skilled emergency clinician. Vascular access can be especially challenging in children who are dehydrated or in shock. This chapter reviews the basic principles and techniques of blood sampling, as well as placement of peripheral and central intravenous (IV) and intraarterial catheters in infants and children, including the use of umbilical catheters in newborns. Also reviewed are hydration techniques for dehydrated children. For critically ill and injured children, intraosseous (IO) access is the preferred technique if peripheral vascular access cannot be secured rapidly (see Chapter 25). Though rarely required, emergency cutdown is occasionally lifesaving, and a section of this chapter is devoted to cutdown techniques. Fear and anticipation of pain associated with procedures or injections make the hospital experience traumatic for children. Before beginning any painful procedure in a child, explain the procedure to the parents, as well as the reasons that it needs to be done. For children capable of understanding, explain the procedure in developmentally appropriate language before starting and before each successive step. Avoid using deceptive phrases such as “this won’t hurt.” A gentle, honest explanation that the procedure will hurt a bit and a statement such as “it is okay to cry, but not to move” will provide realistic expectations for the child and set limits as well. Depending on the situation, most parents wish to remain with their child during the procedure.1 Others will not. The potential for parents to faint at the sight of blood or needles should always be addressed, and it is preferable that they sit down during the procedure. If present, the parent’s role should be to provide comfort to the child but not to assist in any potentially painful procedure. If possible, distract the child with simple conversation regarding school, friends, hobbies, pets, or TV shows to help decrease the child’s anxiety. Many products are available to decrease the pain associated with vascular access. Do not delay attempts at access in critically ill or injured children to use these medications or devices, but consider using them in stable patients. These medications and products are discussed in more detail in Chapter 29. Options include vapocoolants, topical anesthetics such as lidocaine-prilocaine (EMLA), 4% liposomal lidocaine (LMX-4), and injection of lidocaine via a needleless jet injector (J-tip). Orally administered sucrose solution (Sweet-Ease) has been demonstrated to decrease the pain response in young infants during procedures.2,3 Procedural sedation is commonly used during central venous and arterial cannulation in children (Chapter 33). Capillary blood sampling is frequently used to obtain blood samples from young infants. In infants, the heel is the most common location for capillary blood sampling, whereas in older children and adults, blood samples are more commonly obtained from the finger, toe, or earlobe. This technique is useful when repeated measurements such as blood glucose or serial hemoglobin are needed. It is also an option for obtaining “arterialized” blood for blood gas analysis when arterial access is unavailable, such as in chronically ill neonates and young infants or when the clinician is not comfortable obtaining a percutaneous arterial blood sample. If a sufficient volume is obtained, blood from a capillary sample can also be sent for other routine laboratory studies. Do not send this type of specimen for blood culture because of the risk for contamination. Heel sticks are more painful than venipuncture but are useful in the event of difficult access or when arterialized samples are needed.4 The heel stick method of capillary blood sampling will be described, but capillary blood samples can also be obtained from a finger, toe, or earlobe (Fig. 19-1). To avoid penetration of the calcaneus and the risk for osteochondritis, puncture only the most medial and lateral portions of the plantar surface of the heel.5 Some research suggests that when using automated lancets, any site on the heel can be safely punctured in a term neonate.6 Prewarm the foot for 5 minutes to produce hyperemia and enhance blood flow. Immobilize the foot in a dependent position with one hand. First cleanse the heel with antiseptic solution and allow it to dry. Next, puncture the skin with the lancet. Allow the alcohol to dry to avoid false elevations in the glucose level. Avoid squeezing the foot because it may inhibit capillary filling and actually decrease the flow of blood. Furthermore, squeezing may lead to hemolysis and make analysis less accurate. If blood does not flow freely, another puncture may be required. When performed properly, heel sticks are associated with a low incidence of complications. Use a proper incision device to prevent lacerations. Rare complications include infection, scarring, and calcified nodules.7 As in adults, the usual site for venipuncture in infants and children is the antecubital fossa. However, any reasonably accessible or easily visible peripheral vein may be used, such as those on the hands, feet, or scalp for very small infants (Fig. 19-2). Veins on the dorsum of the hand can be used, provided that they will not be needed for IV cannulation. The external jugular and femoral veins or arterial sites are rarely needed for routine samples in a stable patient. Imaging devices (e.g., ultrasound, transillumination, or infrared devices) may also be used to locate and identify veins for IV catheter placement. These devices are discussed later in this chapter (see “Vascular Line Placement: Venous and Arterial”). Cleanse the area surrounding the chosen site of skin penetration with antiseptic solution and allow it to dry. Apply slight distal traction to the skin to immobilize the vein. Insert the needle quickly through the skin and advance it slowly into the vein at an angle of approximately 30 degrees with the bevel facing up (Fig. 19-3, step 1). Successful vessel penetration is heralded by a flashback, or flow, of blood into the butterfly tubing. Apply gentle suction by slowly withdrawing the plunger of the syringe. If the required amount of blood is greater than the capacity of the attached syringe, pinch off the tubing, remove the filled syringe, attach a new syringe, and apply gentle suction again after releasing the pinched tubing. After the required amount of blood is withdrawn, remove the needle and apply a sterile dressing and direct pressure to the puncture site. Apply suction with a Vacutainer system in which the needle punctures the sealed end of the butterfly device (see Fig. 19-3, step 2). There are also butterfly systems available with a second needle that is occluded by a rubber shield located at the opposite end of the butterfly tubing. Once the vein has been entered, the needle at the opposite end of the butterfly tubing is pushed through the top of the vacuum-sealed tube. In either case, if the suction is excessive, the vein will collapse and blood flow will stop. Although peripheral sites for venous blood sampling are preferable in infants, the external jugular and femoral veins may also be used for venipuncture during resuscitation or when peripheral sites are inadequate. The external jugular vein lies in a line from the angle of the jaw to the middle of the clavicle and is usually visible on the surface of the skin (Fig. 19-4). When the infant is crying, this vein is more prominent. Ask an assistant to restrain the infant in a supine position with the head and neck extended over the edge of the bed. Alternatively, place a towel roll or pillow under the child’s shoulders. Turn the head approximately 40 to 70 degrees from the midline. Cleanse the skin surrounding the area to be punctured with alcohol or another antiseptic solution. Apply finger pressure just above the clavicle to help distend the jugular vein. Use a 21- to 25-gauge straight needle or a 21- to 25-gauge butterfly needle attached to a syringe. Puncture the skin and then advance the needle slowly until the jugular vein is entered and a flashback of blood is observed. Keep the syringe connected to the needle at all times to maintain constant negative pressure and avoid air embolism. After the appropriate amount of blood is obtained, withdraw the needle and apply slight pressure to the vessel. Place the infant in an upright position after the needle is removed, and hold pressure over the puncture site for 3 to 5 minutes. Observe the puncture site closely afterward to identify persistent bleeding. In most patients, the femoral vein lies medial to the femoral artery and inferior to the inguinal ligament (Fig. 19-5A). Ask an assistant to position the patient’s hips in mild abduction and extension while you palpate the artery. Identify its location by placing a mark on the skin just superior to the femoral triangle. If available, use ultrasound to assess the position of the femoral vessels. Prepare the femoral triangle with alcohol or another antiseptic agent. Use a povidone-iodine or chlorhexidine scrub when obtaining blood for culture. Use a technique of needle insertion that is similar to that for external jugular venipuncture (see Fig. 19-4). Puncture the skin and then direct the needle or catheter toward the umbilicus at a 30- to 45-degree angle to the skin and just medial to the pulsation of the femoral artery (see Fig. 19-5B). Apply slight negative pressure constantly throughout insertion. After the needle enters the femoral vein, withdraw the desired blood samples. Afterward, remove the needle or catheter unless an IV catheter for venous access is desired in this location. Apply pressure over the puncture site in the femoral triangle for a minimum of 5 minutes. Observe closely for recurrent bleeding. Scalp veins can be very useful for venous sampling in small infants when other options are not possible or readily available.10 The anatomic considerations and technique are discussed later (see “Peripheral Venous Catheterization: Percutaneous” and “Peripheral Venous Catheterization: Venous Cutdown”). Indications and Contraindications The radial artery has several advantages that make it the most commonly used artery for blood sampling. First, its location makes it easy to palpate and puncture (Fig. 19-6A). The ulnar artery is more difficult to locate. Second, no vein or nerve is immediately adjacent to the radial artery, which minimizes the risk of obtaining venous blood or damaging a nerve. Another advantage of the radial artery is the presence of good collateral circulation from the ulnar artery. The brachial artery has little collateral circulation and should be avoided unless no other options are available.11 Limit use of the ulnar artery to preserve collateral circulation to the hand. As a general rule, do not use the femoral artery for obtaining routine blood samples. The radial artery is the one most frequently used vessels to obtain intermittent arterial samples, so the technique for arterial puncture at this site will be described. (See also Chapter 20 for a discussion of the Allen test and the effect of heparin on arterial blood sampling.) Hold the infant’s wrist and hand in your nondominant hand (see Fig. 19-6B). Hold the hand fully supinated with the wrist slightly extended (i.e., dorsiflexed). Palpate the arterial pulsation just proximal to the transverse wrist creases. Do not overextend the wrist because this can cause loss of the arterial pulse during palpation. Make a small indentation in the skin with a fingernail to mark the insertion site. Cleanse the area with antiseptic and allow the skin to dry. The topical anesthetic options discussed previously may be used if the clinical situation permits. Penetrate the skin at a 30- to 45-degree angle. While the plunger of the syringe is gently withdrawn by an assistant, advance the needle slowly until the radial artery is punctured or resistance (bone) is met (Fig. 19-7). In contrast to performing the procedure in adults, provide continuous, but gentle suction with the plunger of the syringe in infants. Pulsating or rapidly flowing blood that appears in the hub of the needle is a good indication that the radial artery has been punctured. Some clinicians prefer to attach the syringe to the butterfly needle only after blood return is noted. Suction can be applied afterward. Complications of radial artery puncture include infection, hematoma formation, arterial spasm, tendon injury, and nerve damage.12,13 With proper technique, however, the complication rate is extremely low. If the infant starts to cry before blood is obtained, the Po2 and Pco2 values may not reflect the infant’s true steady state. A variety of imaging modalities, including ultrasound, transillumination, and infrared technologies, have been used to help locate peripheral veins for cannulation. In adults there are data supporting the use of ultrasound to facilitate peripheral vein cannulation in those with difficult access.14,15 However, the use of ultrasound to aid in the placement of peripheral IV lines in pediatric patients is not common practice. One recently published study demonstrated that ultrasound could be used to detect peripheral veins in young children that were not “clinically apparent” (nonvisible and nonpalpable).16 The study also found that lack of ultrasound visualization increased the chance of unsuccessful placement.16 Other small studies have shown mixed results, and further research is needed.17–19 Some emergency departments (EDs) use transillumination devices commonly found in neonatal intensive care units to assist in finding veins in infants.20,21 The Venoscope II (Venoscope LLC, Lafayatte, LA) and the Neonatal Transilluminator (Graykon Scientific, Victoria, Australia) are two such devices that work by projecting a high-intensity light into the patient’s subcutaneous tissue. The light causes the veins to contrast with the surrounding tissue, which makes them easier to locate.22 A newer vein imaging technology, VeinViewer (Luminetx Corp., Memphis, TN) uses near-infrared technology to project an enhanced image of the subcutaneous veins onto the patient’s skin. Theoretically, knowledge of the location of the venous valves and the course of the vessel can assist the clinician in selecting the best area to be cannulated.23 Early small studies have not demonstrated improved overall success rates in obtaining IV access, but the technology may be useful in patients with difficult IV access.24–26 A number of IV sites are available for placement of a peripheral IV line in an infant (see Fig. 19-2). The most common sites chosen for IV insertion in infants and children are the superficial veins of the dorsum of the hand, the antecubital fossa, the dorsum of the foot, and the scalp (in newborns and small infants). The veins of the dorsum of the hand are the vessels most frequently used. Because these veins are relatively straight and lie flat on the metacarpals, they are easily stabilized. If the hand is chosen, take the child’s age and hand preference into consideration. Avoid placing an IV line in a hand used for thumb sucking whenever possible. Veins in the antecubital fossa (cephalic and basilic veins) are easily accessible; however, the angulation across the fossa makes advancement of the catheter difficult. These veins may not be easily visible and yet may be palpable. Select the most distal vein that is large enough to accommodate the catheter and leave the larger, more proximal veins in case the initial attempts are unsuccessful or if prolonged IV therapy is needed and percutaneous central venous catheter placement (e.g., a peripherally inserted central catheter line) is being contemplated. Tributaries of the dorsal venous arch on the dorsum of the foot, like those on the dorsum of the hand, are relatively straight, and the extremity is easily immobilized after insertion. Because indwelling catheters in this location prevent mobility, consider using this site only in preambulatory patients or after attempts at other sites have been unsuccessful. The scalp veins are easy to cannulate, but their use is primarily limited to very small infants. If a peripheral vein on the hands, feet, or antecubital fossa is being used, immobilize the extremity first by taping it to an arm board, a padded splint, or a commercially available immobilization device. The particular site is a matter of preference, so choose the vein that appears to be the easiest to cannulate. With few exceptions, the same techniques used for IV insertion in adults may be used in infants and children, especially in the veins of the distal ends of the extremities. If the peripheral end of an extremity is used, place a tourniquet proximal to the planned site of entry. Warm the extremity to induce vasodilation in the surface veins, which makes them easier to cannulate. Flush the tubing of the T-extension set before venipuncture with a sterile IV solution, such as normal saline (NS), to prevent air embolism. Direct the IV catheter through the skin at a 10- to 20-degree angle and slowly advance it until blood return is noted (Fig. 19-8, step 1). Next, advance the catheter over the needle and into the vein. Retract the needle and connect the IV line to the hub of the catheter by means of a T-extension set (see Fig. 19-8, step 2). After 1 mL of saline has been flushed through the line, inspect the site for signs of infiltration, such as hematoma or local swelling. Figure 19-8 Pediatric peripheral intravenous catheterization. (See Figure 21-6 for additional details on proper intravenous technique.) Fix the catheter to the skin with a 0.5-inch piece of tape passed over the catheter hub and skin. Place a second piece of tape adhesive side up and slip it under the catheter hub. Cross it over the catheter hub in a V shape (Fig. 19-8, step 2). After securing the catheter with tape, cover the entire area with a transparent sterile dressing such as Tegaderm (3M, St. Paul, MN) or OP Site (Smith and Nephew Medical, Massillon, OH). Loop back the tubing of the T-extension set, place a piece of tape midway over the tubing, and secure it to the skin. This ensures that the IV tubing will not be accidentally dislodged if is suddenly pulled. Securely tape the hand and forearm to an arm board for immobilization (see Fig. 19-8, step 3). Occasionally, the flow rate of the infusion may depend on the position of the catheter, especially if the catheter spans a joint or the tip abuts a venous valve. Adjust the hand position or catheter with strategically placed sterile gauze or withdraw the catheter slightly to remedy the problem. If the scalp veins are used, trim the hair in the surrounding area to expose the vessels. To differentiate between arteries and veins on the scalp, arteries are usually more tortuous than veins.10 If an artery is entered during placement of the needle and fluid is infused, blanching will occur in the area. If this happens, remove the IV catheter, maintain slight pressure for 5 minutes, and repeat the procedure at another site. A rubber band may be used as a tourniquet around the scalp to produce venous dilation, but it is rarely required. Place a piece of tape on the rubber band before placement on the scalp to facilitate lifting the rubber band away from the scalp. If a scalp vein butterfly infusion set is used, grasp the wings of the butterfly between the thumb and forefinger and introduce the needle beneath the skin approximately 0.5 cm distal to the anticipated site of vein entrance (Fig. 19-9, step 1). Advance the needle slowly toward the vessel until blood appears in the tubing, which indicates that the vessel has been entered. Next, remove the tourniquet. Flush the needle with 0.5 to 2 mL of IV fluid, such as NS, to ensure that the needle is properly in place within the vein. If infiltration occurs, as noted by a subcutaneous bump, remove the IV line and repeat the process at another site. After the wings are secured, tape the tubing of the butterfly set in a loop on the scalp so that it is not pulled inadvertently. Place a wisp of cotton under the wings of the butterfly if the flow rate of the infusion is affected by the position of the catheter. Tape a small medication cup over the wings and the needle to protect the IV line (see Fig. 19-9, step 2). Connect the tubing of the butterfly set to the tubing from the IV system. It is generally preferable to use standard over-the-needle IV catheters whenever possible, whether for extremity or scalp IV lines, because they are less likely to infiltrate and will last longer. However, a small butterfly needle that can be inserted temporarily until additional access is possible is sometimes the only option short of IO access. External Jugular Venous Catheterization: The external jugular vein is superficial and easily visible. It can be used when other attempts at peripheral IV access have been unsuccessful. The external jugular vein is undesirable as a primary catheterization site during resuscitative efforts because manipulation of the head and neck may compromise management of the airway. Moreover, it is not a suitable site for central venous catheterization because of the acute angle of entry of the external jugular vein into the subclavian vein.27 Technique.: Because central venous access is not the goal of this approach, the external jugular vein is most often entered with a standard over-the-needle IV catheter. The external jugular vein lies in a line from the angle of the jaw to the middle of the clavicle and is usually visible on the surface of the skin. The vein is more prominent when the child is crying. Ask an assistant to restrain the patient in a supine position with the head and neck extended over the edge of the bed. Alternatively, place a towel roll or pillow under the shoulders. Turn the head approximately 40 to 70 degrees from the midline (see Fig. 19-4). Cleanse the skin surrounding the area to be punctured with alcohol (or another antiseptic solution). Cover the area with a sterile drape, and infiltrate 1% lidocaine into the skin. Place a finger just above the clavicle to distend the jugular vein. Indications and Contraindications With the development of small IV catheters and the rapidity and safety of IO needle placement for emergency access (Chapter 25), peripheral venous cutdown is rarely performed in the ED. Even in experienced hands a saphenous vein cutdown may take more than 10 minutes and is associated with a higher rate of infection than other routes of vascular access are.28 Nevertheless, if peripheral venous, central venous, or IO access cannot be obtained, venous cutdown may provide an alternative means of emergency venous access. For the purpose of illustration, exposure and cannulation of the saphenous vein will be discussed (Fig. 19-10). The same principles apply when cutdown is performed on most peripheral veins.
Pediatric Vascular Access and Blood Sampling Techniques
Patient Preparation and Restraint
Anesthesia
Blood Sampling Techniques
Indications and Contraindications
Technique
Complications
Venipuncture
Technique
Arterial Blood Sampling
Technique
Complications
Vascular Line Placement: Venous and Arterial
Peripheral Venous Catheterization: Percutaneous
Vein Imaging Devices
Technique
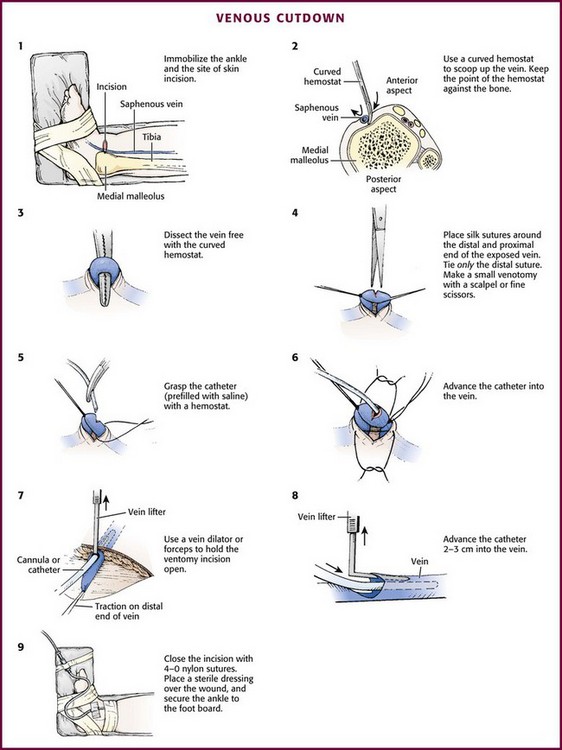
Peripheral Venous Catheterization: Venous Cutdown

 , 1, and 2 inches)
, 1, and 2 inches)