Chapter 100Pathophysiology and Clinical Diagnosis of Cortical and Subchondral Bone Injury
Clinical Examination
Clinical signs of cortical bone injury include periosteal thickening and local sensitivity. This is easily recognized in horses with the bucked-shin complex38-40 (see Chapter 102). However, in the majority of long bones, overlying soft tissue and muscle prohibit clear identification. Localized signs of cortical pain in the tibia or humerus are uncommon and frequently absent.16,25,28,31 When noted, pain during palpation and percussion of the medial diaphysis of the tibia occurs in as few as 30% of affected horses.25 Careful palpation may reveal focal pain in horses with avulsion fracture of the proximal palmar or plantar aspect of the McIII or the MtIII, respectively.41 Pain on palpation or profound muscle spasm of the affected side and ventral displacement of a tuber sacrale are consistent but subtle physical abnormalities in horses with stress fractures of the wing of the ilium.15,30 Flexion tests, time-honored clinical tools to help exacerbate and localize lameness, often have inconclusive results, and findings may even be absent in horses with cortical bone injury.24,42
Clinical signs of subchondral bone injury are also variable and often subtle. Injuries are frequently bilateral, and overt unilateral limb lameness is unusual. Affected horses may simply be performing at a lower level than expected. Overlying cartilage is minimally affected early in the disease process, and joint effusion is absent. Early subchondral bone injury of the distal aspects of the McIII and the MtIII rarely causes fetlock effusion,36,43-45 and carpal effusion is often less than expected based on severity of lameness in Standardbreds (STBs) with subchondral lucency of the C3.46,47 Diagnostic analgesia is essential for accurate diagnosis, but perineural techniques are consistently more effective than intraarticular analgesia, presumably because pain is associated with subchondral bone and not synovitis, capsulitis, and overt cartilage damage (see Chapter 42). Veterinarians and trainers are often incredulous when a diagnosis of subchondral bone injury is made by use of diagnostic analgesia and scintigraphic examination when clinical signs are lacking. As with cortical bone injury, flexion test results are inconsistent and often negative in horses with subchondral bone injury.36,46 Subchondral increased radiopacity may be radiologically apparent, but it is often difficult to determine whether this is successful adaptive change or a maladaptive or nonadaptive response. In those horses with severe or chronic subchondral bone injury, clinical signs are often more obvious. Synovitis, lameness, and OA may be noted.
Diagnostic Analgesia
Although diagnostic analgesia is one of the most valuable tools used to localize the authentic source of pain, there are several considerations in horses with stress fractures and subchondral bone injury. At the time of evaluation, horses must be lame enough to visually assess response to diagnostic analgesia. Horses with stress fractures are notorious for being lame immediately after intense exercise but subtly lame or sound at the time of lameness examination. Neither perineural nor intraarticular analgesia can be used to diagnose upper limb stress fractures; however, such fractures should be suspected when lameness cannot be improved by routine distal limb diagnostic analgesia.* In addition, response to diagnostic analgesia is often variable. Lameness associated with cortical or subchondral injury of the proximal palmar aspect of the McIII may improve with high palmar analgesia or intraarticular analgesia of the middle carpal joint.27,41,42,50 Pain associated with nonadaptive subchondral remodeling of the distal aspect of the McIII or the MtIII may be alleviated by using a low palmar or plantar or the lateral palmar metacarpal or plantar metatarsal block.51 Often these horses will not improve with intraarticular analgesia and are similarly unresponsive to intraarticular medications.36,43,44 Diagnostic analgesia is a good starting point but must not be used alone.
Diagnostic Imaging
Radiography and Radiology
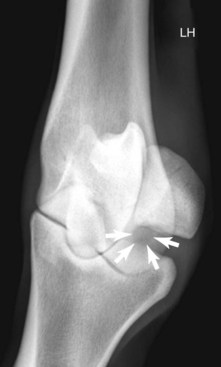
Radiography remains hugely important in the diagnosis of bone injury in racehorses; however, it is insensitive during early phases of stress-related bone injury and often inadequate for subtle subchondral changes. Radiology provides structural information, much of which must be several weeks old before it can be seen. Lag time for enough structural change to occur to be seen radiologically is an obviously serious limitation of radiography, especially when one is attempting to localize and identify early or subtle bone injury. It takes 2 to 3 weeks or more before radiological changes of periostitis are apparent (see Chapter 15).52-55 Even frank fracture lines or compression injury of subchondral bone can take days to weeks to be recognized. Supplementary radiographic images of site-specific regions may enhance detection of injury. For example, the dorsal 30° proximal 45° lateral-palmar (or plantar) distal medial oblique and dorsal 30° proximal medial-palmar or plantar distal lateral oblique (“down-angled”) images (Figure 100-1) are useful for evaluation of subchondral injury of the condyles of the McIII or the MtIII.51,55 A dorsal 60° proximal 45° lateral-palmar distal medial oblique image or dorsal 60° proximal 45° medial-palmar distal lateral oblique image of the distal phalanx is necessary for identification of palmar process fractures.56-58 Even with additional images, subtle subchondral radiolucency and increased radiopacity are often difficult to recognize because density differences must be at least 30% to 50% and lesions 1 to 1.5 cm in diameter before recognition is possible.52 Another obvious limitation of radiography is the inability to distinguish whether the radiologically apparent lesion is an active process or simply the result of an adaptive bone response.
Nuclear Scintigraphy
Increased awareness of location and prevalence of stress-related bone injuries and scintigraphic examination of affected racehorses enhance early detection of bone pathology before catastrophic fracture. Nuclear scintigraphy is an extremely sensitive method of detecting exercise-induced bone remodeling (see Chapter 19). Normal bone response in the dorsal aspect of the McIII, the proximal sesamoid bones (PSBs), and the metacarpal condyles is easily identified and has typical patterns of increased radiopharmaceutical uptake (IRU) in horses undergoing high-speed exercise.59,60 Scintigraphy is useful to detect abnormal alterations in local bone metabolism such as increased activity from cyclic loading and provides a foundation for the diagnosis of stress fractures, stress remodeling, and subchondral trauma.* A focal, moderate-to-intense area of IRU is the scintigraphic hallmark of stress fracture.
Ultrasonography
Ilial wing fractures can be detected ultrasonographically.15,17,30,66,67 Hematoma formation, irregular bone contour (indicative of callus formation), and breaks in the normal hyperechogenic contour of bone may be seen if the fracture involves the dorsal surface of the bone (see Figure 49-3, page 566). A displaced fracture is seen as hyperechogenic bony structure distracted from adjacent bone (“stair-step” sign). Ultrasonographic abnormalities are usually most severe at the caudal margins of the fracture.66,67 These findings are supported by postmortem studies in horses with ilial wing fractures.11,13 Serial ultrasonographic examinations can be used to determine if the fracture has healed but should not be the single means of diagnosis. Because of variability in conformation of the ilium and other factors, ultrasonographic findings should be combined with a thorough clinical examination and, if necessary, scintigraphic and occasionally radiographic examinations.15,66
Specific Locations of Cortical and Subchondral Bone Injury
Knowledge of the prevalence of stress-related bone injuries provides insight into the likelihood of an area causing clinically important problems, especially when diagnostic analgesia fails to localize the lameness, or radiological and ultrasonographic examination findings are unremarkable. The type of training and racing largely determines the location of injury and whether the injury involves cortical or subchondral bone (Tables 100-1 and 100-2). In lame STBs, IRU is most commonly associated with exercise-induced subchondral bone remodeling, and prevalent sites include the PSBs followed by the C3 and the tarsus.63 The distal palmar or plantar aspect of the McIII or the MtIII is also a frequent location of IRU and, when combined with IRU of the PSB, is the most common location of stress remodeling.55,63 In TBs, IRU of the distal palmar or plantar aspects of the McIII or the MtIII is the most common abnormal scintigraphic finding.62 In the forelimbs, sites of prevalence are the distal palmar aspect of the McIII, followed by the carpus and the dorsal cortex of the McIII.62 In the hindlimbs, the distal plantar aspect of the MtIII is the most common abnormal area, followed by the tarsus and tibia.62
TABLE 100-1 Prevalences of Sites of Increased Radiopharmaceutical Uptake in the Forelimbs of Racehorses
| SITE | STANDARDBRED (%) | THOROUGHBRED (%) |
|---|---|---|
| Phalanges | 14 | 10 |
| Proximal phalanx | 7 | — |
| Middle phalanx | 0 | — |
| Distal phalanx | 7 | — |
| Proximal sesamoid bone | 32 | 6 |
| Third metacarpal bone | ||
| Distal palmar | 21 | 50 |
| Dorsal | 0 | 15 |
| Proximal palmar | 3 | 4.5 |
| Carpus (C3) | 43 |