Chapter 54Pathogenesis of Osteochondrosis
Osteochondrosis: Definitions and Terminology
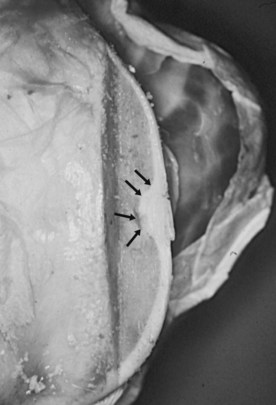
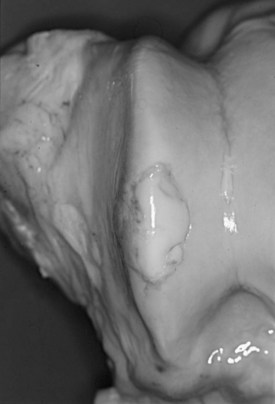
Equine osteochondrosis (OC) is characterized by focal failures of endochondral ossification that typically occur in well-defined predilection sites. Lesions that result from external trauma or infection are not generally regarded as OC.1,2 The thickened, retained growth cartilage that characterizes typical lesions (Figure 54-1) in the articular-epiphyseal cartilage complex (AECC) may be complicated by development of fissures that extend from the deepest layers of the lesion to the articular surface. Cartilaginous or osteochondral fragments may then detach from the parent bone, forming intraarticular fragments.3 Once lesions extend to the articular surface, thereby causing inflammation of the joint, the condition may be referred to as osteochondritis.4,5 The term osteochondritis dissecans (OCD) is usually reserved for lesions in which a dissecting flap of tissue is present (Figure 54-2).3 Although it has been proposed that the term dyschondroplasia should be used in place of OC,3,6 the term OC remains in widespread use and is used in this chapter to refer to the primary lesion. Dyschondroplasia is used only when referring to work by authors who prefer this term. A condition similar to equine OC occurs in a number of other animal species, as well as in people.1
Endochondral Ossification
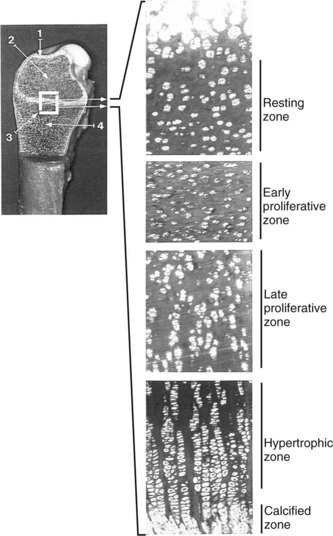
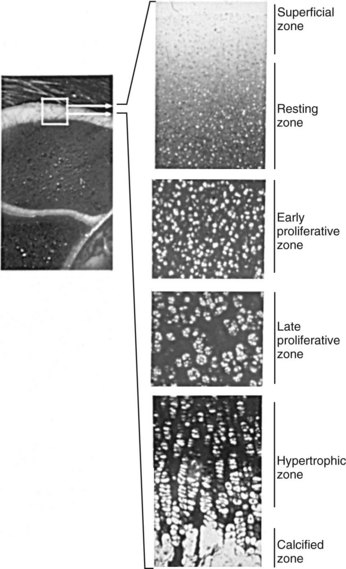
Endochondral ossification is the process by which growing cartilage is systematically replaced by bone to form the growing skeleton.7 This process occurs at three main sites: the physis, the epiphysis, and the cuboidal bones of the carpus and tarsus. Chondrocytes in the physis can be divided into a series of layers or zones (Figure 54-3). The zone farthest from the metaphysis is the resting or reserve zone. Adjacent to this is the proliferative zone, in which chondrocytes divide. These cells progress to the hypertrophic zone, in which they enlarge and form ordered columns. During this stage the chondrocytes become surrounded by extracellular matrix that gradually becomes mineralized in the zone of provisional calcification. The chondrocyte columns are then invaded by metaphyseal blood vessels, and bone forms on the residual columns of calcified cartilage. This mixture of calcified cartilage and immature bone (primary spongiosa) is then gradually remodeled to produce the mature bone of the metaphysis.7 Endochondral ossification, which continues throughout the period of growth, also occurs in the AECC at the ends of long bones (Figure 54-4).8 The chondrocytes of the AECC that are closest to the articular surface produce articular cartilage, whereas those cells closer to the epiphysis participate in endochondral ossification in the same manner as occurs in the physis. It is generally accepted that the growth cartilages of both the physis and the AECC are susceptible to OC.1,3,8-11
Characteristics of Osteochondrosis
It is obvious from this short summary that histological definitions and descriptions of OC vary, making accurate identification of lesions difficult.24 Even chondrocyte clusters (Figure 54-5), considered by many to be one of the most consistent findings in OC lesions,24,26 are not pathognomonic for the disease,15,24 and, unless used to define the condition, necrosis is not a universal finding.25,27 It is thus not surprising that the relevance to OC of some of the lesions studied has been questioned.28 The matter is complicated further by the limited repertoire of responses that bone and cartilage can mount to injury and developmental abnormalities12 and by the fact that many lesions are not identified until they have reached the chronic stage. The features of such chronic lesions may represent the results of secondary change and attempted repair, rather than primary osteochondrotic change. The results of studies based on such lesions may thus be misleading. This situation has resulted in a wide-ranging, heterogeneous, and somewhat confusing body of literature, in which equine OC has been ascribed to genetic, dietary, endocrine, biomechanical, traumatic, ischemic, and toxic causes.12,18,26,29-33
Relationship among Physeal Dysplasia (Physitis), Subchondral Bone Cysts, and Osteochondrosis
The relationship between OC and physeal dysplasia (physitis or epiphysitis) is poorly defined. Retained cartilage has long been regarded as a possible cause of physeal dysplasia,16,34 and OC lesions of the physis have been described in clinically affected horses (thickened and irregular metaphyseal growth cartilage)5,10 and in foals with experimentally induced dyschondroplasia (retained cartilage cores).26,35 If OC truly is a generalized or multifocal disturbance of endochondral ossification, it would seem logical that metaphyseal growth cartilage has the potential for involvement. However, some have questioned whether articular and physeal lesions have the same cause and have proposed that physitis be regarded as a manifestation of developmental orthopedic disease but not of OC.12 Moreover, the results of a recent study suggest that many enlargements of the distal aspect of the third metacarpal and metatarsal bones, including those with evidence of increased metaphyseal cartilage thickness, represent physiological remodeling rather than a pathological process.36 The role of OC in the pathogenesis of equine physeal dysplasia thus remains unclear. However, it should be noted that a similar pathogenic mechanism (failure of blood supply to growth cartilage) has been proposed for both articular OC and physeal lesions in pigs.1 Physitis is covered in more detail in Chapter 57 and is not discussed further here.