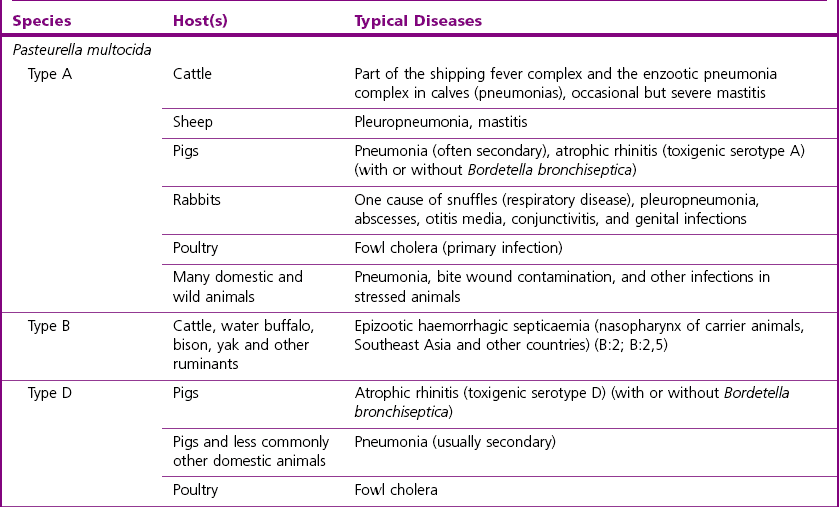
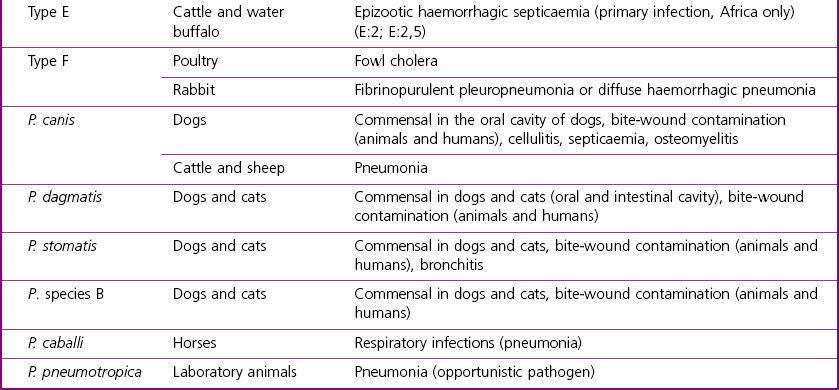
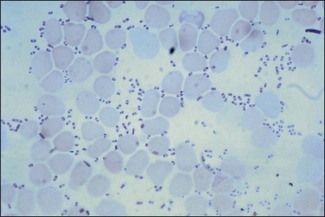
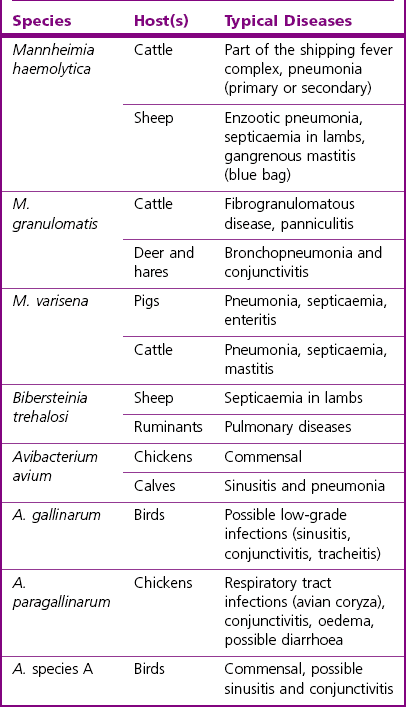
Chapter 21 Classification of organisms in the genus Pasteurella is revised regularly and the taxonomy of the genus is complex. Pasteurella sensu stricto contains P. multocida, P. canis, P. stomatis, P. dagmatis, and P. species B. Pasteurella caballi and P. pneumotropica are both species of uncertain taxonomy, while P. avium, P. gallinarum, P. volantium and P. species A have all been reclassified under the genus Avibacterium (Blackall et al. 2005). Haemophilus paragallinarum has been reclassified as Avibacterium paragallinarum while P. trehalosi has been reclassified into the genus Bibersteinia (Blackall et al. 2007). M. haemolytica, formerly classified as P. haemolytica A serotypes (biogroup 1), is the main species of veterinary significance within the Mannheimia genus, followed by M. granulomatis and M. varigena. Pasteurella, Mannheimia, Bibersteinia and Avibacterium species are worldwide in distribution with a wide spectrum of hosts. They have a predilection for the oral and respiratory tracts of animals and/or humans. Most are commensals on the mucous membranes of the upper respiratory and/or intestinal tracts of animals, humans and birds. The carrier rate for different species varies greatly. Many of them are opportunistic pathogens and cause disease only under certain conditions. Interestingly, P. multocida can survive in water for a year as well as in organic material for long periods (Bredy & Botzler 1989). The principal hosts and diseases associated with Pasteurella, Mannheimia, Bibersteinia and Avibacterium species are listed in Tables 21.1 and 21.2. Isolates of the genera Pasteurella and Mannheimia cause a wide variety of diseases of great economic importance, mainly in poultry, pigs, cattle and rabbits. The review by Kehrenberg et al. (2001) provides a short summary of the infections caused by Pasteurella and Mannheimia isolates in food-producing animals. Infections caused by Pasteurella and Mannheimia species may be endogenous or exogenous. The portal of entry is usually via the respiratory tract and virulence is enhanced by animal-to-animal transmission, as occurs in pneumonic pasteurellosis. Subcutaneous infections are usually the result of animal bites. Various stresses, including concurrent viral infections, predispose to infection, as occurs in ‘shipping fever’. Table 21.2 Main diseases caused by the major pathogenic Mannheimia, Bibersteinia and Avibacterium species in veterinary medicine The main virulence factors of P. multocida and M. haemolytica are described in Table 21.3. Endotoxins (lipopolysaccharides, LPS) are particularly important in the septicaemic diseases such as fowl cholera and bovine haemorrhagic septicaemia. Pasteurella multocida serotyes A and D can produce a cytotoxic protein named P. multocida toxin (PMT), which stimulates cellular cytoskeletal rearrangements and growth of fibroblasts. Interestingly, avirulent PMT-positive strains and virulent PMT-negative strains have both been reported (Rimler & Brogden 1986). However, PMT plays a role in atrophic rhinitis (mild to severe destruction of porcine nasal turbinate bones), while co-infection with toxigenic strains of Bordetella bronchiseptica promotes colonization by P. multocida. Iron acquisition genes have been described in P. multocida (Bosch et al. 2002) which facilitate the removal of iron from the host and promote bacterial growth. The polysaccharide capsule of P. multocida offers protection against the host immune system by reducing macrophage uptake and diminishing susceptibility to the bactericidal activity of complement. Capsular adherence properties have also been described (Pruimboom et al. 1996). The composition of the capsule can vary from one serotype to another. Many fimbrial subunits and filamentous haemagglutinins have been described and are thought to play a role in adhesion to eucaryotic host cells (Al-Haddawi et al. 2000, May et al. 2001). Table 21.3 Main virulence factors of Pasteurella multocida and Mannheimia haemolytica M. haemolytica serotype A1 produces a heat-labile leukotoxin, a member of the RTX (Repeats in ToXins) toxin family, with cytotoxic cell specificity for bovine leukocytes (Shewen & Wilkie 1982, Highlander 2001). This toxin acts by forming pores in leukocytes resulting in cell lysis. This virulence factor is considered of major importance in M. haemolytica A1 infections. Mannheimia haemolytica also secretes a sialoglycoprotease (metallo-endopeptidase) which cleaves the sialoglycoproteins on the surface of epithelial cells, macrophages and leukocytes (Abdullah et al. 1992). Therefore, this enzyme may have a role in adhesion and colonization of the respiratory tract by M. haemolytica. In addition, its ability to cleave bovine IgG1 may reduce the effectiveness of the immune response (Mellors and Lo 1995). The LPS of M. haemolytica has endotoxic activity (stimulates release of pro-inflammmatory cytokines, induces microvascular necrosis and thrombosis). The capsule has been reported to prevent phagocytosis, mask the cell surface, promote resistance to complement and aid adherence to host cells. Other virulence factors include fimbriae which facilitate adherence and a siderophore which promotes iron acquisition. Neuraminidase is involved in the removal of sialic acid residues from host glycoproteins, thus promoting adherence, while superoxide dismutase (metallo-enzyme) plays a role in the detoxification of free radicals. The small, Gram-negative rods or coccobacilli are not always readily discernible in Gram-stained smears from affected tissues. In septicaemic conditions, such as fowl cholera, distinctive bipolar-staining Pasteurella multocida can be seen in Giemsa or Leishman-stained smears (Fig. 21.1).
Pasteurella, Mannheimia, Bibersteinia and Avibacterium species
Genus Characteristics
Natural Habitat
Pathogenesis and Pathogenicity
Bacteria
Virulence determinants
Functions
P. multocida
PMT toxin (serotypes A and D)
Cytotoxic protein that stimulates cell cytoskeleton rearrangements
DnaK, DnaJ, DjlA, HscA, HscB, HtrA, GrpE, HslV, HslU, GroES, GroEL
Heat shock proteins
Capsular polysaccharide
Prevent phagocytosis, resistance to complement, adherence
SodA, SodC, Tpx, HktE, TsaA, ThdF
Detoxification
Iron uptake system genes tonB, exbD and exbB
HemB, HemE, HemH, HemL, HemU, Fur, RsgA1, RsgA2
Iron acquisition
SurA, SurE
Stationary phase survival
Filamentous hemagglutinins (PfhB1 and PfhB2), surface fibrils (Hsf_1 and Hfs_2), and fimbrial subunits (PtfA, FimA, Flp_1, Flp_2)
Adhesion to host cells, chemotaxis
M. haemolytica
LPS
Endotoxic activity, stimulates release of pro-inflammmatory cytokines, microvascular necrosis and thrombosis
Leukotoxin (pore forming cytolysin)
Cytotoxic to ruminant leukocytes
Capsule
Prevent phagocytosis, mask cell surface, resistance to complement, adherence
Fimbriae
Adherence
Siderophore
Iron acquisition
Neuraminidase
Removal of sialic acid residues from host glycoproteins, thus promoting adherence
Metallo-endopeptidase (sialoglycoprotease)
Cleavage of sialoglycoprotein on the surface of epithelial cells, macrophages or leukocytes
Superoxide dismutase (metallo-enzyme)
Detoxification of free radicals
Laboratory Diagnosis
Direct microscopy
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pasteurella, Mannheimia, Bibersteinia and Avibacterium species
Only gold members can continue reading. Log In or Register to continue