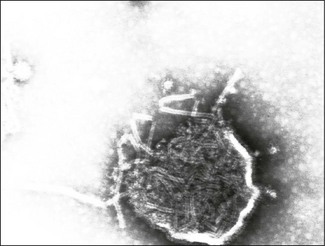
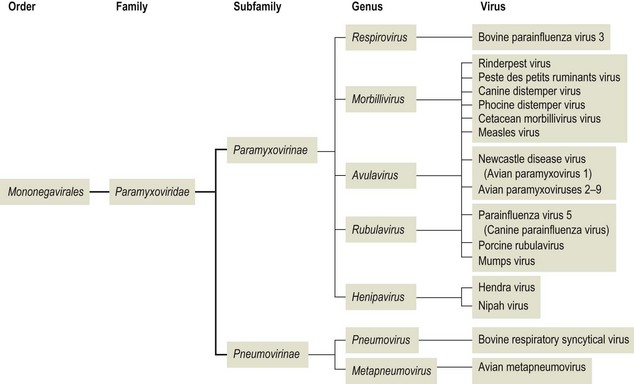
Chapter 61 Orthomyxoviruses and paramyxoviruses were originally grouped together and known as the ‘myxoviruses’. The name paramyxovirus is derived from two Greek words, para meaning by the side of and myxa meaning mucus. Paramyxoviruses are pleomorphic though usually spherical in shape, 150 to 200 nm in diameter and enveloped (Fig. 61.1). The envelope is covered in glycoprotein spikes of two types, an attachment protein (G or H or HN) and a fusion protein (F). These glycoproteins are responsible for interaction with host cell surface receptors and for fusion of host cell membrane and virus envelope, respectively. They are of primary importance in eliciting virus-neutralizing antibodies and inducing immunity to reinfection. There is also an envelope-associated non-glycosylated matrix (M) protein. Paramyxoviruses can possess haemagglutinating, haemolytic and neuraminidase activities. The nucleocapsid has helical symmetry, is non-segmented, 13 to 18 nm in diameter, has a characteristic ‘herringbone’ appearance and has a single molecule of linear, negative-sense, single-stranded RNA. Replication occurs in the cell cytoplasm with release by budding from the plasma membrane at sites containing virus envelope proteins. Virions are labile, being sensitive to heat, desiccation, lipid solvents, non-ionic detergents and many disinfectants. The families Paramyxoviridae, Rhabdoviridae, Filoviridae and Bornaviridae contain enveloped viruses with genomes consisting of a single molecule of negative-sense, single-stranded RNA and together form the order Mononegavirales. The family Paramyxoviridae has undergone several taxonomic changes including the creation of three new genera, Metapneumovirus, Henipavirus and Avulavirus and the renaming of the genus Paramyxovirus as Respirovirus. The family is divided into two subfamilies, Paramyxovirinae and Pneumovirinae (Fig. 61.2). Members of the Paramyxovirinae are usually capable of haemagglutination and have been grouped in five genera; Respirovirus, Rubulavirus, Henipavirus, Avulavirus and Morbillivirus. The subfamily Pneumovirinae contains two genera; Pneumovirus and Metapneumovirus. Paramyxoviruses are genetically stable and do not undergo recombination. The only mechanism permitting some degree of variation is mutation. Paramyxoviruses infect vertebrate hosts, mainly mammals and birds, and usually have a narrow host range (Table 61.1). Transmission occurs via close contact or aerosol. Primary replication typically occurs in the respiratory tract. Infection is generally cytolytic but temperate and persistent infections also occur. Syncytium formation and intracytoplasmic, acidophilic inclusions are characteristic features of infection. Morbilliviruses are capable of inducing intranuclear, acidophilic inclusions. The family contains several members responsible for the production of serious disease in animals and man. Paramyxoviruses have been shown to be of significance in wild animal populations. A number of new morbilliviruses including phocine distemper virus and cetacean morbillivirus have been discovered in marine mammals. Hendra virus was isolated following an outbreak of severe respiratory disease in horses in a stable in Brisbane, Australia, in 1994. Two in-contact humans were also affected. Fourteen horses and their trainer died. In 1999 a related virus, Nipah virus, was isolated in Malaysia following large outbreaks of disease in pigs and their human handlers. More than 100 human deaths due to a febrile encephalitic illness were recorded. Both viruses are capable of infecting cats and dogs. Human-to-human spread does not appear to occur. Fruit bats of the genus Pteropus are the natural hosts of both viruses. Table 61.1 Paramyxoviruses of veterinary significance Rinderpest or cattle plague is an acute disease of cloven-hoofed animals that has been recognized for many centuries as a devastating disease of cattle and domestic buffalo. Epizootics of the disease in Europe provided the catalyst for the setting up of veterinary schools and state veterinary services as well as the foundation of the Office International des Epizooties (OIE), also known as the World Organization for Animal Health. It is classed as a listed disease by the OIE. A global eradication scheme by the Food and Agriculture Organization (FAO) of the United Nations (UN), begun in 1994, was officially declared a success in 2011. A number of factors made this a realistic goal including a single serotype, an excellent vaccine that confers lifelong immunity, good diagnostic tests, and the absence of a carrier state. On the basis of genetic sequencing, rinderpest virus isolates can be grouped into three distinct evolutionary lineages: African lineages 1 and 2 and Asian lineage 3. Towards the end of the eradication campaign, two reservoir sites of infection, the Indus River buffalo tract in Pakistan and the Somali pastoral ecosystem of southern Somalia and northern Kenya, were recognized (Roeder & Taylor 2002). The last clinical case was recorded in Kenya in 2001. In enzootic areas or following the laboratory confirmation of an epizootic the clinical and pathological findings may be sufficient for a diagnosis. However, in regions where rinderpest is uncommon or absent laboratory confirmation is required to differentiate the condition from conditions such as bovine viral diarrhoea and malignant catarrhal fever. A pen-side test based on a rapid chromatographic strip test has been reported to be a useful tool for field personnel investigating outbreaks (Bruning et al. 1999). Specimens should be collected from several febrile animals early in the disease. • Lesions are characteristically found throughout the alimentary tract. The longitudinal folds of the colonic and rectal mucosae often appear highly engorged with bloody streaks creating the appearance of ‘zebra striping’. The base of the tongue, retropharyngeal lymph node and third eyelid are suitable tissues for histopathology and immunohistochemistry. Syncytia and intranuclear inclusion bodies may be evident in sections. Rinderpest virus antigens can be demonstrated using immunohistochemical staining. • Suitable specimens for isolation of virus include the buffy coat from uncoagulated blood collected from pyrexic animals. Alternatively, 20% suspensions (w/v) of spleen, prescapular or mesenteric lymph nodes may be used. Specimens should be kept cool on ice and not frozen, followed by rapid transport to the laboratory. The use of glycerol as a preservative should be avoided as it inactivates the virus. Suitable cell lines include primary calf kidney and Vero cells. Rinderpest virus produces cytopathic effects in cell culture and its presence can be confirmed by immunofluorescence. • Agar gel immunodiffusion or a counter-immunoelectrophoresis test are suitable as simple, rapid antigen detection assays (Foreman et al. 1983). The test is carried out at 4°C using anti-rinderpest rabbit hyperimmune serum. Suitable specimens include ocular secretions and exudates from the cut surface of spleen or lymph nodes. The test is not particularly sensitive or specific but is robust and easy to perform. • A reverse transcription-polymerase chain reaction (RT-PCR) method capable of detecting and differentiating between rinderpest virus and peste des petits ruminants virus has been developed (Forsyth & Barrett 1995). Three primer sets are required; two ‘universal’ sets based on conserved regions of the phosphoprotein and nucleoprotein genes of morbilliviruses and a rinderpest virus-specific primer set based on fusion protein gene sequences. • An immunocapture ELISA has been described for the rapid differentiation of rinderpest virus and peste des petits ruminants virus (Libeau et al. 1994). • A competitive ELISA for the detection of serum antibodies to rinderpest virus has been developed and adopted by the OIE as the prescibed test for international trade (Anderson et al. 1991). The test is based on competition between antibodies in positive sera and a rinderpest anti-H protein monoclonal antibody for binding to rinderpest antigen. The virus neutralization test (Plowright & Ferris 1961) is considered the ‘gold standard’ serological test but is not as easy or cheap to perform (Roeder & Taylor 2002). Peste des petits ruminants or goat plague is an acute contagious disease of small ruminants, particularly goats. It is caused by peste des petits ruminants virus (PPRV), closely related to other members of the morbillivirus genus. Isolates of PPRV can be grouped into four distinct lineages on the basis of partial sequence analysis of the fusion protein gene (Dhar et al. 2002). The disease occurs in sub-Saharan Africa north of the equator, the Middle East, India and Pakistan. In West Africa, epizootics are associated with the onset of the rains when flocks are gathered together and surplus kids are sold. Infection rates are similar in sheep and goats but disease is generally more severe in goats. It is an OIE listed disease. • Specimens for laboratory confirmation should be obtained during the acute phase of the disease and include nasal and ocular swabs, unclotted blood and scrapings of buccal and rectal mucosae. Pieces of lung, spleen, mesenteric and bronchial lymph nodes and intestinal mucosae may also be collected from animals killed early in the course of the infection. • Rapid antigen detection methods include immunocapture ELISA (Libeau et al. 1994), counter-immunoelectrophoresis and agar gel immunodiffusion. A rapid and inexpensive haemagglutination assay has been described for PPRV (Wosu 1991). • Specific primers, designed to amplify the fusion and nucleocapsid protein genes (Forsyth & Barrett 1995, Couacy-Hymann et al. 2002), have been successfully applied in the RT-PCR technique. This technique has been shown to be very sensitive. • Virus isolation may be carried out in primary lamb kidney or African green monkey kidney (Vero) cell lines. Blind passages may be required before CPE, consisting of cell rounding, aggregation and syncytia formation, becomes evident. • Serological detection of antibodies can be carried out by virus neutralization or competitive ELISA (Libeau et al. 1995).
Paramyxoviridae
Virus
Host species
Disease
Rinderpest virus
Cloven-hoofed animals, particularly cattle and domestic buffalo
Highly contagious disease responsible for devastating outbreaks characterized by high morbidity and high mortality. First animal virus to be eradicated globally
Peste des petits ruminants virus
Small ruminants, particularly goats
Severe disease with many similarities to rinderpest. High morbidity and mortality rates seen in epizootics in goats. OIE listed disease
Bovine parainfluenza virus 3
Cattle, sheep
Produces subclinical or mild respiratory disease. Associated with shipping fever. Predisposes to secondary bacterial infection, particularly Mannheimia haemolytica. Worldwide distribution
Bovine respiratory syncytial virus
Cattle, sheep, goats
Common infection in cattle, worldwide. Associated with moderate to severe respiratory disease outbreaks in young cattle
Porcine rubulavirus
Pigs
Cause of blue eye disease. Only described in Mexico to date
Canine distemper virus
Wide range of domestic and wild carnivores
Worldwide, acute infection of dogs characterized by multisystemic involvement and moderate mortality
Parainfluenza virus 5 (Canine parainfluenza virus)
Dogs
Inapparent or mild respiratory disease in dogs. May be involved in kennel cough outbreaks
Feline morbillivirus (FmoPV)
Cats
Newly discovered virus of cats. Possible association with tubulointerstitial nephritis
Newcastle disease virus (Avian paramyxovirus 1)
Wide range of domestic and wild bird species including chickens, turkeys, pigeons, pheasants and waterfowl
Cause of Newcastle disease. Varying virulence isolates designated velogenic, mesogenic and lentogenic. Generalized infection characterized by respiratory, intestinal and/or nervous signs. OIE listed disease
Avian metapneumovirus
Turkeys, chickens
Associated with coryza in turkeys and swelling of sinuses (swollen head syndrome) in chickens
Rinderpest
Diagnosis
Peste des petits ruminants
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Paramyxoviridae
Only gold members can continue reading. Log In or Register to continue