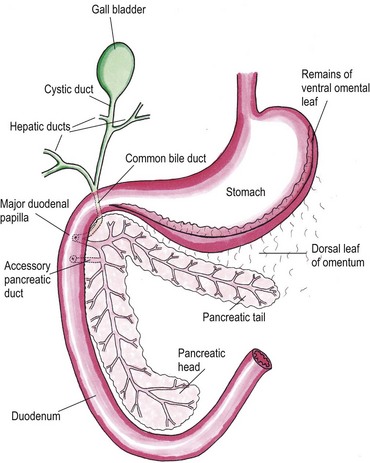
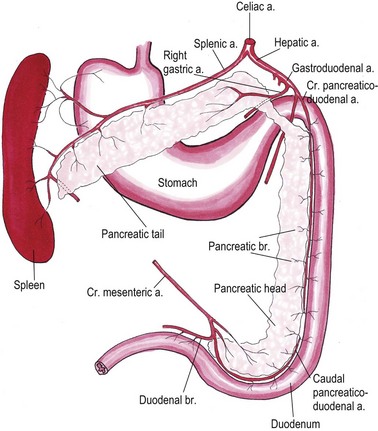
Chapter 33 The pancreas develops from the dorsal and ventral primordium in the hepatopancreatic ring of the duodenum and grows into the dorsal omentum. It has a long and slender configuration in the cat, taking the shape of a wide V or boomerang. The right or duodenal lobe is located in the mesoduodenum and often takes a hook-like shape at the end.1 It is best accessed surgically by gentle traction on the descending duodenum ventrally and medially. The left or splenic lobe runs in the dorsal mesogastrium towards the dorsal extremity of the spleen and has an omental connection to the mesocolon. For surgical visualization the omentum and stomach need to be retracted ventrally, or alternatively the omental pouch can be opened by tearing the ventral leaf. Both pancreatic lobes unite in the pancreatic body close to the cranial part of the duodenum and pylorus (Fig. 33-1). The normal pancreas has a pale pink color and is finely lobulated. The exocrine portion of the pancreas is a compound tubuloacinar gland whose ducts are totally concealed within the substance of the organ.2 Interlobular and interlobar ducts unite to form the main pancreatic duct that opens into the duodenum on the major duodenal papilla together with the common bile duct. In about 20% of cats, a second smaller duct, the accessory pancreatic duct, opens approximately 10 mm aborad on the minor duodenal papilla (Fig. 33-1).3 A pancreatic bladder consisting of aberrant pancreatic tissue with a dilated pancreatic duct is described in 0.2% of cats.4 It lies beside the gall bladder with which it can communicate. It is regarded as a pathologic variant without clinical significance. Rarely, accessory nodular pancreatic lobes are found along the feline gut, especially in the area of the duodenum, and in the portal mesentery. These often extend towards the hepatic fossa.4 The endocrine part of the pancreas consists of several thousand pancreatic islets of Langerhans and accounts for approximately 2% of the gland’s weight.5 Pancreatic α- and β-cells produce glucagon and insulin, respectively, which regulate carbohydrate homeostasis. δ-Cells secrete the growth inhibiting hormone somatostatin and PP cells produce pancreatic polypeptide that self regulates pancreas secretion activities and affects hepatic glycogen levels and gastrointestinal secretions. The exocrine portion of the gland consists of acinar and ductal cells. Acinar cells are arranged in clusters around the terminal pancreatic ductules.6 They primarily secrete digestive enzymes: proteases (trypsinogen, chymotrypsinogen, proelastase, procarboxypeptidase, ribonucleases, and deoxyribonucleases), which cleave polypeptide chains; amylase and lipase, which hydrolyze carbohydrates and fat; and trypsin inhibitors. To prevent pancreatic autodigestion, enzymes are either synthesized, stored and secreted as inactive zymogens (proteases) and activated within the intestinal tract,7 or an inactive form is directly secreted and activated within the duodenum (pro-phospholipase A2) and/or they need cofactors like bile salts and colipase for function (phospholipase A2, lipase, carboxylesterase). Amylase is secreted intact and active.8 Ductal cell secretions contain bicarbonate and water that serve to neutralize pH in the duodenum, intrinsic factor that facilitates cobalamin (vitamin B12) absorption in the distal ileum, and antibacterial proteins that regulate small intestinal bacterial flora.7 In cats, the pancreas is the only source of intrinsic factor, whereas in dogs intrinsic factor is also secreted by the stomach. The pancreatic arterial supply derives mainly from the celiac artery: the splenic artery contributes branches to the left pancreatic limb and the cranial pancreaticoduodenal artery and right gastric artery give radicles to the cranial part of the right pancreatic limb. The caudal pancreaticoduodenal artery originates from the cranial mesenteric artery and supplies the caudal half of the right pancreatic limb (Fig. 33-2).9 Anastomoses between these various vessels occur within the gland.10 Venous drainage is via portal tributaries. Lymphatic drainage is into the pancreaticoduodenal and hepatic lymph nodes; however, there are lymph vessels draining from the pancreas to the jejunal and splenic lymph nodes.11 A complete physical examination including a thorough evaluation of the cat’s abdomen is mandatory. Cranial abdominal masses and cranial abdominal discomfort are easily overlooked as the clinical response of the cat can be very subtle. Jaundice is a common finding and is usually a sign of complicating cholangiohepatitis, hepatic lipidosis, or biliary obstructions secondary to pancreatic disease. Lethargy, anorexia, dehydration, and hypothermia are the most prevalent clinical signs (Tables 33-1 and 33-2). The cat can dramatically deteriorate with development of shock and progression to a moribund state despite aggressive fluid therapy.12 A minimum database should consist of a complete blood cell count, serum bilirubin, cholesterol, glucose, total protein, albumin, and serum activity of liver enzymes (serum alanine aminotransferase and alkaline phosphatase), calcium, urea, creatinine, and potassium. Common clinicopathologic findings in cats with pancreatic disease are listed in Tables 33-3 and 33-4. Ionized hypocalcemia was found to be a negative prognostic indicator in cats.13 Table 33-4 Clinicopathologic abnormalities in cats with pancreatic neoplasia48,60,66 The measurements of serum lipase and amylase activities do not seem to be of clinical value in the diagnosis of feline pancreatic disease. Several different lipase isoenzymes originate from various cells in the body and circulate in the blood; they are all detected by catalytic lipase assays, thus the tissue of origin cannot be determined. Species-specific immunoassays for the measurement of feline trypsin-like immunoreactivity (fTLI) and feline pancreatic lipase immunoreactivity (fPLI) have therefore been developed and validated. Feline pancreatitis is best detected by the measurement of elevated fPLI.14,15 A summary of specific tests for pancreatic disease is given in Table 33-5. Table 33-5 Pancreas-specific serum tests and their availability fPLI, feline pancreatic lipase immunoreactivity; fTLI, feline trypsin-like immunoreactivity. Lateral and ventrodorsal projections are evaluated for loss of peritoneal detail suggestive of peritoneal effusion or a cranial abdominal mass and subsequent displacement of internal organs into the upper right quadrant of the abdomen. The duodenum often appears displaced and corrugated in traumatic pancreatitis; rarely, calcification in the pancreatic and peripancreatic area is visible.16 In acute cases, radiographs are also useful to rule out other causes of acute abdomen. Ultrasonography is the most useful imaging modality to support the diagnosis of feline pancreatic disease (Fig. 33-3).14,17,18 Soft tissue masses located in the cranial abdomen can be caused by pancreatitis, cysts/pseudocysts, abscesses, or neoplasia. Concurrent abdominal effusions can be readily identified. Acute feline pancreatic disease is associated with a hypoechoic pancreas with hyperechoic peripancreatic fat and thickening of gastric and duodenal walls. More chronic cases with acute flare-ups usually show mixed echogenicity of the peripancreatic tissues. Cats with chronic disease can have a dilatation of the gall bladder and common bile duct.17–19 The use of Doppler ultrasonography has shown promise in preliminary studies.20 Although specificity is high, the overall sensitivity of abdominal ultrasound to detect feline pancreatitis is low (24–35%).17,18 While acute pancreatitis can be identified as described above, chronic pancreatitis does not often produce ultrasonographically visible changes. Depending on the presence of permanent histopathologic changes after resolution of the disease, pancreatitis is classified as acute (no permanent changes) or chronic (irreversible morphological change).21 Acute necrotizing pancreatitis (ANP) is characterized by a predominance of pancreatic acinar cell necrosis and peripancreatic fat necrosis.12 Various amounts of inflammation, hemorrhage, and mineralization can be present.7 Some fibrosis is possible if there is chronic underlying disease with an acute flare-up. The etiopathogenesis of ANP is unknown and it is mostly considered idiopathic; however, various diseases and conditions have known associations with cats developing ANP (Box 33-1). Pancreatitis may be caused by a reduction in pancreatic blood flow due to hypotension under general anesthesia, or due to temporary venous outflow occlusion during surgery.22 It rarely develops secondary to pancreatic biopsy or excision of pancreatic neoplasia.23,24 In acute suppurative pancreatitis, neutrophilic inflammation is the predominant histologic feature. The condition is less common than ANP and appears to affect younger animals.12,13,25,26 There is significant overlap in the various forms of pancreatitis, and ultimately histopathology is needed to distinguish the conditions.25 Pancreatic atrophy is believed to be the consequence and end stage of CP in most feline cases and it may or may not affect the endocrine portion of the gland as well.7 Many cats develop exocrine pancreatic insufficiency, resulting in severe nutrient maldigestion, acid injury to the duodenal mucosa, cobalamin and fat-soluble vitamin malabsorption, and bacterial proliferation in the gut.27 In pancreatitis, it is generally believed that premature trypsin activation of digestive zymogens within pancreatic acinar cells is followed by pancreatic autodigestion and inflammation: acinar cell necrosis, hemorrhage, and fat necrosis and saponification take place.7 Systemic consequences of intracellular enzyme activation are mast cell degranulation, leukocyte chemotaxis, platelet aggregation, vasodilation, surfactant degradation within the lungs and, in severe cases, initiation of disseminated intravascular coagulation (DIC). Under normal conditions, circulating proteolytic enzymes are bound by α-macroglobulins and rapidly cleared. When circulating α-macroglobulins are depleted, proteases activate the kinin, coagulation, fibrinolytic and complement cascades, resulting in the development of acute DIC and death.5 Fluid, acid–base and electrolyte deficits should be corrected. Pancreatitic cats can be hypokalemic and hypocalcemic. Calcium gluconate should be given at a dose of 50–150 mg/kg IV over 12–24 hours if necessary and serum total or ionized calcium concentration should be monitored during therapy. Anemic cats with a packed cell volume below 15–20% are transfused with whole blood or packed red blood cells after blood typing and cross-matching (see Chapter 5). Plasma transfusion may be beneficial to increase available α-macroglobulins that bind circulating active proteases, and to combat DIC. Increased concentrations of albumin will help to maintain plasma oncotic pressure and blood volume and reduce pancreatic edema. A short period of fasting has been recommended in the past especially for dogs with pancreatitis. This should only be extrapolated to the cat in cases of severe vomiting at risk for aspiration pneumonia. With their predilection for the development of fat mobilization and hepatic lipidosis, it is extremely important to feed cats through their bouts of pancreatitis. Esophagostomy, gastrostomy, gastroduodenostomy, or jejunostomy feeding tubes may be placed to provide enteral support in anorexic animals (see Chapter 12). Ideally, small frequent portions of a diet high in carbohydrate and low in fat are given. Aggressive analgesic therapy based on systemic opioids should be provided (Table 33-6). Pain in cats is not easily detected as they tend to exhibit pain by withdrawal (being quiet, purring, hiding), thus severe pain should be suspected in any animal with pancreatitis. Nausea and vomiting in affected cats can be severe and there is a variety of anti-emetics available for use in cats; these are listed in Table 33-6. Septic complications of acute pancreatitis are rare in cats, but broad-spectrum antibiotics should be given if toxic changes are present in the blood or if the patient is febrile. Necrosis and inflammation predispose to colonic bacterial translocation and colonization of the pancreas, mainly with E. coli and other coliforms.28,29 Indications for surgery for pancreatitis include an enlarging or persistent pancreatic mass in a patient unresponsive to medical treatment, and sepsis non-responsive to aggressive medical treatment and extraluminal bile duct obstruction. Abscesses and large necrotic areas are debrided (see Box 33-3), and omentectomy (see Chapter 19) with or without splenectomy (see Chapter 34) is performed if necessary. The abdomen is lavaged with warm sterile saline to remove activated proteases from the abdomen. Hyperbilirubinemia due to extrahepatic bile duct obstruction can occur as a consequence to pancreatitis or pancreatic fibrosis and needs to be addressed surgically if non-responsive to medical treatment. In those instances, a biliary diversion technique such as cholecystoduodenostomy or cholecystojejunostomy (see Chapter 31) can be used. Alternatively, biliary diversion is facilitated by tube cholecystostomy. Recently, choledochal stenting has been advocated in cats to achieve biliary decompression.30 Numbers of operated cats are low and prognosis seems to be less favorable than in the dog. Two of seven cats died directly postoperatively, three more within seven to 24 months and only two survived long term following stenting. An overall mortality rate of 50% for biliary diversion techniques in the cat has been reported,31 so it seems prudent to be very cautious not to operate prematurely.
Pancreas
Surgical anatomy
Diagnosis and general considerations
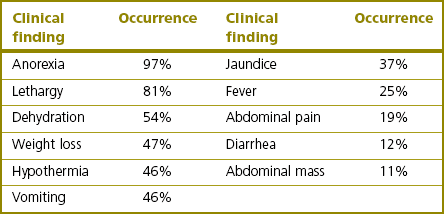
History and clinical examination
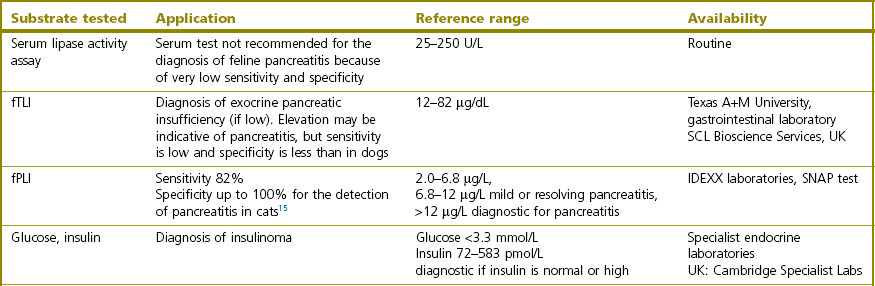
Clinicopathologic tests
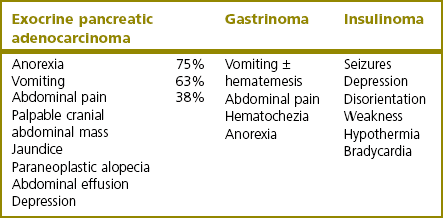
Exocrine pancreatic adenocarcinoma
Gastrinoma
Insulinoma
Leukocytosis
Hyperglycemia
ALT ↑
Regenerative anemia
Leukocytosis
Neutrophilia
ALP ↑
Albumin ↓
Electrolyte abnormalities due to vomiting
Hypoglycemia <3.3 mmol/L
Normal or high insulin level (reference range 72–583 pmol/L)
Diagnostic imaging
Radiography
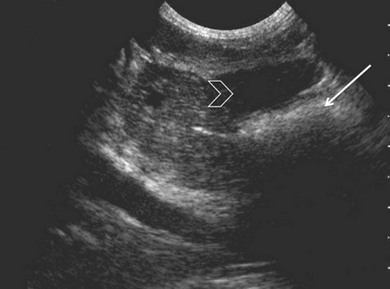
Ultrasonography
Surgical diseases
Pancreatitis
Pathophysiology
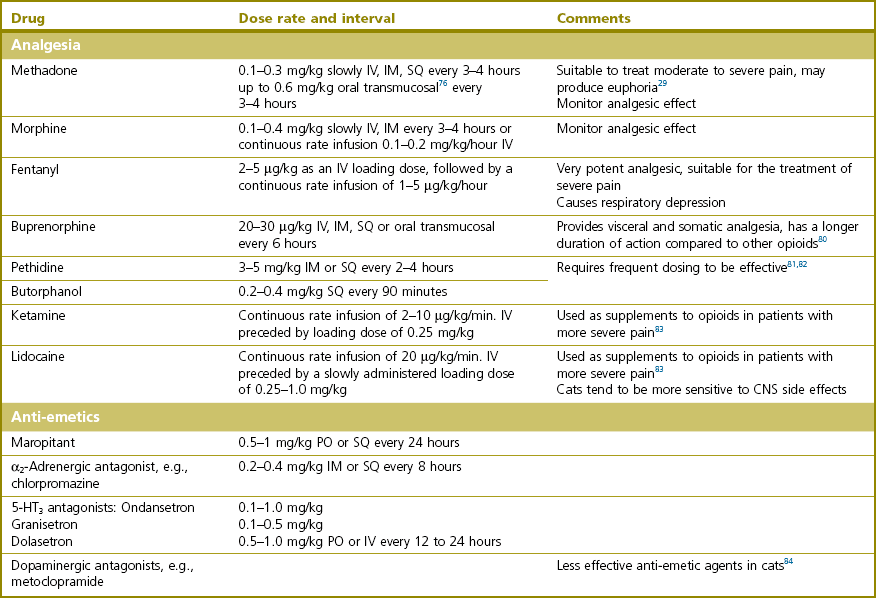
Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pancreas