CHAPTER 1Ovulation and Corpus Luteum Development
The events of ovulation and subsequent formation of the corpus luteum involve systemic and local hormones that signal cellular degeneration and morphogenesis of theca, granulosa, and other associated cells of the preovulatory follicle. Specific cellular aspects of the ovulatory and tissue remodeling processes associated with luteogenesis have been reviewed elsewhere for the mare1,2 and other species3 and will not be discussed herein. Instead, the gross morphologic and physiologic relationships associated with development of the primary follicular wave that leads to ovulation and subsequent formation of the equine corpus luteum, especially during the estrous cycle, will be the primary focus of this chapter. A brief discussion will address the formation of supplemental corpora lutea from ovulatory and anovulatory follicles during early pregnancy in the mare.
Undoubtedly, ultrasound imaging has played a major role in advancing our understanding of ovulation and luteal gland development and is an invaluable diagnostic and prognostic tool for evaluating these events in the mare. Ultrasonographic methods for examining ovarian morphology have been documented in other reviews4,5; therefore the applications and not the technique will be emphasized in this review. The technique of gathering ultrasonographic information will be discussed in more detail only in situations where morphologic characteristics can be used as a measure of physiologic status. We believe that comprehension of the fundamental structural and functional relationships associated with follicular and luteal development will allow the practitioner to diagnose the imminence and occurrence of ovulation and subsequent integrity of the developing corpus luteum to within hours. Quantitative changes in the brightness and darkness of the ultrasonographic gray-scale image (i.e., echogenicity) of the preovulatory follicle, ovulation site, and corpus luteum will be presented as a diagnostic and prognostic aid to allow the practitioner to assess cellular and tissue attributes in real time.
THE OVULATORY FOLLICULAR WAVE
The primary follicular wave of the estrous cycle is a major wave, characterized by a dominant ovulatory follicle and several subordinate follicles.1,2,4–8 A group of 7 to 11 growing follicles greater than 5 mm can be detected ultrasonically at about midcycle of a 22- to 24-day estrous cycle. To distinguish between growing and regressing follicles of the ovulatory wave, more than one ultrasound examination is necessary, especially because atretic follicles of a proceeding minor or major wave may linger and confuse the identity of newly emerging follicles. In regard to the latter, follicular wave dynamics have been critically studied by ablating all follicles greater than 5 mm using ultrasound-guided transvaginal follicle aspiration and inducing luteal regression with prostaglandin-F2α(PGF2α) at 10 days after ovulation. As a result, evaluation of the ovulatory wave can be done with respect to a relatively homogenous population of growing follicles because the population of regressing follicles was removed. The mean diameter profiles of newly emerging follicles and corresponding concentration profiles of systemic hormones of an ablation-induced wave have been compiled from several studies9 and are presented in Figure 1-1 to assist in the following discussion. At the time of wave emergence, the largest follicle usually has a 2- to 3-mm size advantage (approximately 1 day of growth) over other smaller follicles of the wave (see Figure 1-1, A). During the common growth phase, the largest follicle is referred to as the future dominant follicle and the others as future subordinate follicles, because the hierarchal position is often maintained after emergence. Endowed with granulosa cell follicle-stimulating hormone (FSH) receptors, antral follicles of the ovulatory wave develop in response to an increase in circulating concentration of FSH. When the future dominant follicle reaches about 13 mm, peak concentration of the wave-inducing FSH surge is attained and the decreasing portion of the surge begins (see Figure 1-1, B). Concomitantly, circulating concentrations of progesterone are decreasing, while inhibin and luteinizing hormone (LH) are increasing (see Figure 1-1, B and C). The opposing changes in concentrations of FSH and LH during late diestrus/early estrus are attributable, in part, to increasing concentrations of inhibin emanating from the growing follicles of the preovulatory wave and decreasing concentrations of progesterone as a result of a regressing corpus luteum, respectively. During the early stages of this hormonal exchange, the future dominant and subordinate follicles continue growth at a relatively parallel rate (2 to 3 mm/day) until the future dominant follicle reaches 20 to 25 mm at early estrus (Figure 1-1, A). Thereafter, growth of the dominant follicle proceeds unabated, and the remaining subordinate follicles cease growth and eventually regress. The change in growth rates between the dominant and subordinate follicles beginning at the start of the dominance phase is a key event during selection of the ovulatory follicle, and has been termed follicle deviation.6–8 The mechanism associated with follicle selection is not known but appears to involve a developmental advantage of one follicle over another such that the future dominant follicle has an increased capacity for estradiol production, sensitivity to FSH, and specificity to respond to LH through the induction of granulosa cell LH receptors. It appears that subordinate follicles are insensitive to low systemic concentrations of FSH and are not endowed with an abundance of granulosa cell LH receptors. Hence, subordinate follicles regress because of an insufficient ability to respond to subthreshold concentrations of gonadotropins. As reviewed,6 recent studies have shown that intrafollicular concentrations of estradiol, IGF-1, inhibin-A and activin-A increase differentially in the future dominant and subordinate follicles before the beginning of deviation and, therefore, may act as modulators for differentially enhancing the FSH and LH responsiveness of the future dominant follicle. Nonetheless, the developmental disadvantage of subordinate follicles can be circumvented by artificially elevating gonadotropin concentrations before follicle dominance has been established. This is the basis of treatments designed to induce multiple dominant follicles and ovulations using gonadotropin preparations.
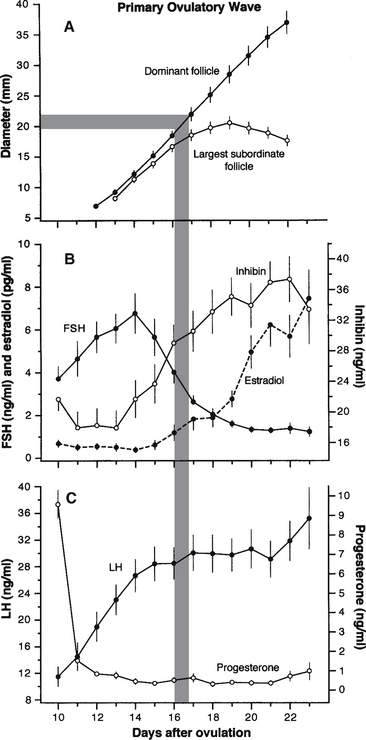
Figure 1-1 Mean (±SEM) follicle diameters and circulating concentrations of hormones associated with development of the primary ovulatory wave are depicted. The data were compiled from several studies9 involving ablation of all follicles greater than 5 mm using ultrasound-guided transvaginal follicle aspiration and induction of luteal regression with prostaglandin-F2α(PGF2α) at 10 days after ovulation in 16 mares. The departure in growth rates between the two largest follicles (follicle deviation) beginning on day 16 when the largest follicle reaches approximately 22.5 mm, on average, represents a key component of the follicle selection phenomenon.6–8SEM, Standard error of the mean; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Continued suppression of FSH during the dominance phase has been attributed to the synergistic effect of inhibin and estradiol. The increase in circulating concentrations of both hormones primarily originate from the developing dominant or ovulatory follicle (see Figure 1-1, B). Apart from its FSH-suppressive effects, high concentrations of estradiol, in the absence of progesterone, appear to exert a stimulatory effect on circulating concentrations of LH.1,2 High concentrations of LH during the latter part of estrus are necessary for continued growth of the dominant follicle, as well as for signaling and preparing the follicle and its contents for ovulation.
OVULATION
Ovulation is a complex process involving a sequence of events1,2 that leads to rupture of a dominant follicle (>30 mm) at the ovulation fossa and the extrusion of follicular fluid, granulosa cells, and the cumulus/oocyte complex. The authors are not aware of any documentation describing ovulation occurring at a site other than the fossa in the equine species. For simplification, spontaneous ovulation occurs as a result of the dominant follicle responding to a rise in circulating concentrations of LH. Under normal physiologic conditions, failure of ovulation of a large dominant follicle may be attributable to inadequate levels of LH or perhaps to an insufficient number of LH receptors (e.g., during early spring and late fall transitional periods, and during diestrus and early pregnancy). Ovulation may be induced artificially or hastened during the transitional and breeding seasons by exogenous LH-like preparations (e.g., human chorionic gonadotropin [hCG]) or LH-releasing preparations (e.g., gonadotropin-releasing hormone [GnRH]) as discussed in Chapter 12 and other reviews.1,2,10,11
Ultrasonographic characteristics associated with impending ovulation of a dominant follicle appear similar whether ovulation is spontaneous or artificially induced. Multiple primary ovulations (synchronous or asynchronous ovulations during estrus) and secondary ovulations (ovulations during diestrus) are considered ovarian irregularities and are discussed in detail in Chapter 13. The following discussion will focus on the characteristics of a single, spontaneous ovulation in association with estrus.
Impending Ovulation
Breeding close to the time of ovulation is an important issue for horse registries that allow only natural mating, for clinics and farms that use cooled or frozen semen, and in instances in which semen quality or quantity is limited as a result of stallion age or morbidity. Ultrasonographic characteristics of the preovulatory follicle have been documented and reviewed.4,5Figure 1-2 was selected from a later study12 to illustrate changes in the ultrasonographic characteristics of the dominant follicle before ovulation.
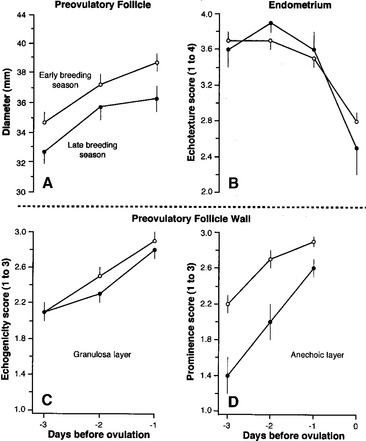
Figure 1-2 Mean (±SEM) ultrasonographic changes in diameter (A) and wall (C, D) of preovulatory follicles and endometrial echotexture (B) 3 days before ovulation during the early breeding season in 32 mares and during the late breeding season in 14 mares as previously reported.12 Follicle growth rate, granulosa layer echogenicity, and endometrial echotexture were not significantly affected by season, but diameter and prominence of the anechoic layer were significantly smaller and less obvious later in the season. Mean follicle diameter and granulosa layer echogenicity and anechoic layer prominence scores were reached the day before ovulation, whereas mean endometrial echotexture scores were reached 2 or 3 days before ovulation. SEM, Standard error of the mean.
Follicle size alone has been the most common and simplest criterion to estimate the time of ovulation. A relatively accurate assessment of size, especially when the follicle has lost its spherical shape, is determined by calculating the average of two lines of measurement from a frozen ultrasound image.4,5,13 Briefly, a line is drawn from border to border of the greatest antral area using the electronic calipers of the ultrasound machine, and a second line is drawn from border to border approximately perpendicular to the first line. Some ultrasound machines have the capability of freezing two different images of the preovulatory follicle side by side. By comparing the quality and area of the two images, the most desirable image may be selected for evaluation.
The mean maximum diameter of the ovulatory follicle is usually between 40 to 45 mm in riding-type mares (Quarter Horse, Arabian, and Thoroughbred),4 but the range can be much greater (e.g., 30 to 70 mm). Maximum follicle diameter can be affected by season (5 to 8 mm larger in the spring versus the summer or fall), breed (approximately 3 mm difference among riding-type mares, 5 mm smaller in miniature, and 10 mm larger in draft mares versus riding-type mares), and multiple ovulations (approximately 4 to 9 mm smaller in double versus single ovulations).4 The effect of animal age on maximum diameter of the ovulatory follicle is equivocal, and the influence of nutrition and parity has apparently not been critically examined. In a study of 181 single ovulations involving riding-type horse mares, none of the follicles ovulated before reaching 35 mm.1 Based on ultrasound examinations done at 24-hour intervals, ovulation occurred a mean of 4.2 days after the dominant follicle first attained 35 mm.1
The mean diameter of the dominant follicle did not change significantly during the 48 hours before ovulation As reviewed,2 and, as illustrated, the rate of growth of the preovulatory follicle slowed 24 hours before ovulation (see Figure 1-2, A). Hence, the maximum ovulatory diameter is not necessarily attained on the day before ovulation; maximum diameter may be attained a day or two earlier. This characteristic was more prominent later than earlier in the breeding season.
If natural mating or artificial insemination with fresh semen is being considered, then daily or every-other-day examinations may be sufficient to estimate ovulation based on size alone. However, insemination is recommended within 12 hours of ovulation (before or after) when using cooled semen, and preferably within 6 hours of ovulation if frozen semen is used.2,10,11 Under these conditions, it may be necessary to shorten the interval between ultrasound examinations and to consider additional criteria to estimate the time to ovulation.
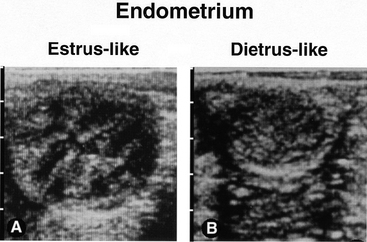
Changes in follicle shape and tone, follicle wall echogenicity (i.e., degree of brightness/darkness of the ultrasound image) and endometrial echotexture (i.e., degree of homogeneity/heterogeneity of the ultrasound image), and sensitivity of the mare to ovarian palpation are additional diagnostic aids that can help to more accurately estimate ovulation to within hours. The change in endometrial echotexture (Figure 1-3) from relatively homogenous during diestrus to relatively heterogeneous during estrus is attributable to an increase in edema of the endometrial folds as a result of the high estrogen:progesterone ratio during estrus.4,5 Changes in endometrial echotexture closely parallel sexual behavior in the mare (e.g., urination, vulvar wink, tail raising, squatting)2 and can be very useful in determining the time to ovulation. The mean duration of estrus during the ovulatory season is 5 to 7 days, but the majority of ovulations (69% to 78%) occur during the last 2 days of estrus, and 10% to 14% occur after the end of estrus.1,2 On average, the degree of endometrial heterogeneity peaks 24 to 48 hours before ovulation and decreases thereafter (see Figure 1-2, B). The change in endometrial echotexture from less estrus-like (see Figure 1-3, A) to more diestrus-like (see Figure 1-3, B) corresponds to the decline in circulating concentrations of estradiol that begins to occur about 1 day before ovulation.1 Typical and atypical characteristics of the uterus during various reproductive states are discussed in more detail in Chapter 5 and other reviews.2,11,14
An increase in the thickness of the follicle wall (i.e., theca layer, basal lamina, and granulosa layer) preceding ovulation has been used as an indicator of impending ovulation.2,4,5 However, in a more critical study,12 ultrasonographic artifacts (e.g., refractory shadows and reverberations) often obscured identification of the antral and stromal borders of the wall. Perhaps a more useful indicator of impending ovulation is the granulosa cell layer alone, which is readily identified by a distinct demarcation between the echoic outer border (basal lamina) and the anechoic inner border (follicular fluid) of the follicle wall (Figure 1-4). Another indicator of impending ovulation may be the appearance of an anechoic layer peripheral to the granulosa layer (Figure 1-4, C and F). Based on daily scanning, mean echogenicity scores of the granulosa layer and prominence of the anechoic layer progressively increased and reached maximal values 24 hours before ovulation (see Figure 1-2, C and D). Prominence of the anechoic layer was more noticeable early in the breeding season than later (see Figure 1-2, D, and Figure 1-4, C and F). Histologic characteristics of the preovulatory follicle led to the interpretation that the increase in echogenicity of the granulosa layer may be due to the increase in the size and number of granulosa cells and that the increase in prominence of the anechoic layer may be due to the increase in size and tortuosity of blood vessels in the theca interna and edema in the theca interna and externa.15 Similar histologic attributes of the preovulatory follicle have been identified in response to hCG.16 In this regard, an anechoic layer in the follicle wall of women has been referred to as a double-contour and reportedly occurs within 8 hours of the LH surge or within a few hours before ovulation.17
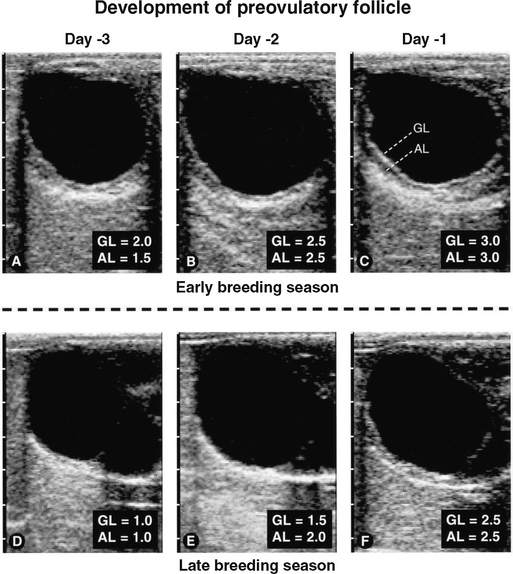
Figure 1-4 Sequential ultrasongrams taken during development of preovulatory follicles 3 days before ovulation in a mare during the early breeding season (A to C) and a mare during the late breeding season (D to F), as previously reported.12 Regardless of season, the granulosa layer (GL) became more echogenic and the anechoic layer (AL) became more prominent and involved more of the circumference of the follicle. As ovulation approached, score values for echogenicity and prominence increased (see Figure 1-2, C and D).
Approximately 12 hours before ovulation, 90% of the dominant follicles become softer or less turgid, 89% change shape from spherical to nonspherical, and mares are more prone to become agitated during palpation of the ovary containing the preovulatory follicle.1,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree