Chapter 61Osteoarthritis
Structure and Function of Normal Joints
Articular Cartilage
Cartilage possesses a number of zones or layers including the following:
The unique functional properties of articular cartilage are reflected in its biochemistry. Articular cartilage is composed of an abundant, specialized extracellular matrix maintained by the aforementioned sparse population of chondrocytes (Figure 61-2). Its water content varies with age but may be as high as 80%.31 This water is freely exchangeable with that in the synovial fluid and is maintained in the matrix in the form of a gel, with matrix collagens and proteoglycans. Water movement is believed to be pivotal to the capacity of cartilage to absorb and distribute compressive load and for its lubrication.

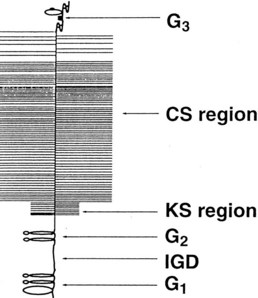
Fig. 61-2 Organization of the major extracellular matrix components in articular cartilage. The principal collagen of cartilage is type II, and a network of these fibrils provides much of the tensile strength of the tissue. Aggrecan is composed of a linear protein with three globular domains (G1 to G3) to which are attached numerous glycosaminoglycan chains of chondroitin sulfate (CS) and keratan sulfate (KS) (see also Figure 61-4). Supramolecular aggregates are formed by the noncovalent interaction of aggrecan with hyaluronan (HA) and stabilized by link protein (Link). The negatively charged glycosaminoglycans (CS and KS) attract several times their weight in water, and this proteoglycan-water composite is responsible for the compressive stiffness of cartilage. Cartilage also has a number of minor proteoglycans and collagens (e.g., decorin and dermatan sulfate [DS], the functions of which are not fully characterized). Fragments of aggrecan, remaining bound to hyaluronan, are depicted to illustrate the effects of proteolytic activity in cartilage.
(From Koopman WJ, editor: Arthritis and allied conditions: a textbook of rheumatology, ed 13, vol 1, Baltimore, 1997, Williams & Wilkins.)
Collagens
Type II collagen is the most abundant in cartilage, accounting for about 90% of the fibrillar network and half of the dry weight of cartilage.2,35 Type II collagen consists of three identical amino acid chains arranged in a triple helix, is less soluble, possesses a higher proportion of hydroxylysine residues, and is more richly glycosylated than type I collagen.36,37 Unlike type I, which typically forms fibers, type II collagen is organized in the form of fibrils that are composed of molecules aligned with a 25% overlap or quarter stagger (Figure 61-3). This structure is stabilized by chemical bonds between specific amino acids in each chain, called hydroxypyridinium cross-links.38 Fibrils are not uniform in size throughout the matrix; they tend to be larger in the middle and deep zones of the matrix, which reflects regional biomechanical demands.39 This protein is arranged in arcades, which form the three-dimensional network or skeleton of the cartilage matrix. Type II collagen is produced by the chondrocytes, and whereas significant degradation and resynthesis of fibrils occur during growth and development, limited turnover occurs in adults.40,41
Proteoglycans
Aggrecan is the primary proteoglycan of articular cartilage that interacts with hyaluronan to form aggregates (see Figures 61-2 and 61-3; Figure 61-4). The individual or monomeric form of this molecule consists of a linear core protein interrupted by three globular domains. The first of these globular domains is designated G1, exists at the amino-terminal portion of the molecule, and is the site at which the proteoglycan attaches to hyaluronan. As many as 100 aggrecan monomers may be attached to the same hyaluronan chain to form supramolecular aggregates of micrometer dimensions (see Figure 61-2).46 The interaction of aggrecan with hyaluronan is noncovalent but is stabilized by a link protein that binds to the G1 domain and hyaluronan with equal affinity.47 Equine link protein was characterized and is similar to that found in human cartilage.48 The specific functions of the G2 and G3 domains are unclear; however, because the G3 domain is present in only about one third of the aggrecan monomers in adult cartilage, it is unlikely that it plays a pivotal role in the extracellular matrix.49
In the region between the second and third globular domains, glycosaminoglycan chains of variable length and composition are attached radially to the protein core (see Figure 61-4). Immediately adjacent to the G2 domain is a region rich in keratan sulfate, and this portion of the proteoglycan, detectable by monoclonal antibodies, has served as a tissue marker of matrix turnover.50 Farther peripherally on the core protein is the chondroitin sulfate–rich region, where up to 100 chondroitin sulfate chains may be found attached radially to the core protein. These chondroitin sulfate chains vary in length, which is the main reason for heterogeneity in the size of aggrecan. Importantly, these glycosaminoglycan chains contain numerous carboxyl and sulfate groups, so that aggrecan is highly negatively charged and can bind up to 50 times its weight in water.46,51,52 This highly hydrated matrix gives cartilage its compressive stiffness and ability to dissipate load.
Intraarticular Volume and Pressure
Intraarticular volume varies and is influenced by joint position (see Chapter 66). Specifically, volume and pressure are respectively minimal and maximal near the extremes of flexion and extension.78-81 This effect is exacerbated in horses with synovial effusion, providing a physiological rationale for diagnostic flexion tests in equine lameness examinations. Moreover, the pointing of an equine limb in which there is joint effusion likely parallels the observation that in the human knee there is a maximum of intraarticular volume (and minimum of intraarticular pressure and pain) at 30 degrees of flexion.82,83
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 ” fragments. Other proteases (e.g., stromelysin) degrade collagen in nonhelical regions.
” fragments. Other proteases (e.g., stromelysin) degrade collagen in nonhelical regions.