Chapter 33 Orthopedics in Small Mammals
Evidence or outcome-based surgery currently does not exist in veterinary medicine, yet it is a major goal of the surgical college in the near future.2,23 Even the human orthopedic college struggles with the fact that many treatments used for common orthopedic conditions are those derived from case experience and not from good clinical trials.
Initial Fracture Management/First Aid
With the exception of ferrets, the small-mammal species commonly kept as companions are prey species. This means that they react to stress and pain differently than do dogs and cats. They may exhibit a state of tonic immobility and not show evidence of pain, even in reaction to severe injury.32 It is important to assess these animals for indicators of shock (see Chapter 38) and neurologic function and to treat them for pain and stress with antianxiolytics (e.g., midazolam), as well as opioids (e.g., butorphanol or buprenorphine). These treatments may make it possible to perform a thorough physical examination as well as to provide additional supportive care (e.g., catheter placement, bandaging).
In handling all open fractures, wear sterile gloves and use aseptic technique, even if the wounds are grossly contaminated. Clip the area around the wound carefully to avoid tearing the delicate skin. A surgical clipper is the best tool for removing fur. Be patient and take care not to further complicate the wound. Carefully debride the wound and lavage the area copiously with warm sterile saline. We recommend the use of a 35- or 60-mL syringe with an 18-gauge indwelling catheter attached to a three-way stopcock and a saline drip set for lavage and debridement, as this combination provides a pressure of 7 to 8 psi to remove debris without damaging tissues.10,49,52 However, this may not be appropriate for very small patients (<200 g); for these patients, gentle lavage may be indicated. Take care not to chill the animal, as small patients lose body heat very quickly. Remove underpads as they become saturated and provide warmth in the form of recirculating water blankets or gel packs. After lavage and debridement, appropriate wound care for the condition of the wound must be instituted.17
Ideally, take swab samples from the fracture site for aerobic and anaerobic bacterial cultures and then start broad-spectrum antibiotic therapy.49 Parenteral formulations are preferred if it is unknown how well the gastrointestinal tract is functioning.
Amputations
Fortunately, most small mammals seen in practice will tolerate amputations. The level of amputation depends on the lesion site. It is very important to cover the amputation site with sufficient soft tissue to prevent trauma to the limb and self-mutilation. After surgery, provide extra cage padding and monitor the animal for foot lesions caused by uneven weight bearing. Because postoperative pain may induce mutilation of the amputation site, consider analgesia in planning these procedures. Use epidural analgesia and local nerve blocks where indicated, either preemptively or intraoperatively (see Chapter 31).
Fracture Fixation Methods
Rabbits have a lower skeletal mass (8%) than cats (13%),11 yet rabbits have a haversian bone structure, like that of dogs and cats.34 Therefore, although the fracture healing process is similar, cortical bone is thinner in rabbits than in dogs or cats. Small rodents (mice and rats) have a primitive bone system; therefore, bone healing is somewhat different in them than in rabbits.34 Because guinea pigs, ferrets, chinchillas, and other rodent species discussed in this book are not used as animal models of bone healing, there is little to no information on the degree of haversian system development in these species.
Many of the surgical implants that veterinarians are accustomed to using are too large for these small species. Even the smallest bone plates available from Synthes Vet (West Chester, PA; http//www.synthesvet.com) can be too large. Although several other companies make bone plates for animals, the smallest screw size, a 1.5-mm cortical screw, is often greater than 40% of the diameter of the bone and therefore too large for use.25 Because they should not exceed 25% of the bone diameter, the positive profile pins that are used with external skeletal clamp systems are too large for use in small mammals.43 Available implants are discussed under the individual headings.
External Coaptation
External coaptation in small mammals can be used effectively as a definitive orthopedic treatment for closed fractures. These methods work best for simple fractures affected by bending and rotational forces because they often interdigitate with manipulation and remain reduced. Fractures affected by compressive or shear force, such as short oblique fractures, are poorly immobilized with external coaptation.13
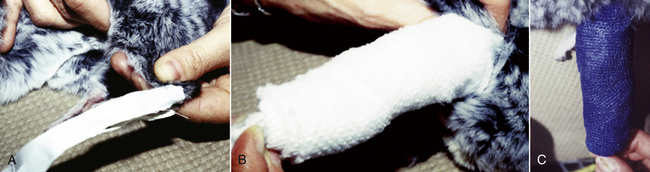
Animals will have to be anesthetized or heavily sedated to allow for proper alignment and prevent further injury. In trying to reduce a fracture in these small animals, take care not to damage soft tissues and the blood supply. External coaptation does not achieve rigid fixation; therefore maintaining the blood supply is paramount to ensure healing. For a good result, it is important to achieve good alignment of the joints and at least 50% cortical contact visible from two radiographic views. Apply standard splinting principles: maintain the affected limb in normal position, immobilize the joint above and below the fracture, and use a bandage and splint that can maintain adequate reduction until bony union (Fig. 33-1). At each splint change, radiograph the limb after placing the splint to ensure adequate reduction. Once the fracture has developed a soft callus with inherent stability, the limb will not have to be radiographed after each change.
Standard materials used for splints and casts in dogs and cats can be applied in managing fractures of rabbits, ferrets, and other small mammals. Several products work well for splinting small mammals, such as Orthoplast (Johnson & Johnson, New Brunswick, NJ), and Vet-lite (Runlite SA, Micheroux, Belgium; http://www.runlite.com/vet-lite/). These are materials that are very lightweight, thin, easy to use, and biomechanically strong. They are malleable when heated in boiling water and conform to any shape when molded and allowed to cool. These products work best when used in a half cast with a light modified Robert Jones bandage, avoiding excessive pressure on the limb. Although these materials cost more than routine casting materials, the final cost is reasonable because only a small piece of material is used. Vet-lite can be layered so that splint thickness and stiffness can be increased if desired. These products are very resistant to chewing by the animal and will not cause oral trauma or break apart and cause gastrointestinal obstruction. Encircling fiberglass casts are difficult to place without causing pressure sores and irritation, but they can be used with care. Fiberglass casting material can be used in strips to make a splint, or they can be bivalved; however, the plastic materials, like Orthoplast, are easier to use and maintain.
Monitor animals with splints and casts weekly to prevent complications. Common complications include chewing at the splint; soiling, which leads to the formation of pressure sores or local skin infections; and swelling, which results from excessive activity or from the splint being too tight. Joint laxity and stiffness from the coaptation are also seen.50
Intramedullary Pinning
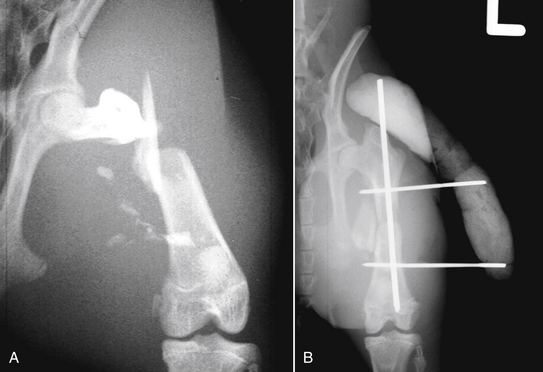
Intramedullary (IM) pinning can be an effective method of internal fixation for small mammals. The small diameter K wires (pin diameters of 0.9-1.6 mm) will fit many of these species. Ideally, the intramedullary pin should fill about 70% of the diameter of the medullary cavity. In one rabbit study, an IM pin that was 75% of the diameter of the bone caused a fracture when being inserted.2a Using a pin that is less than 70% of the bone diameter is unlikely to efficiently control bending unless an external skeletal fixator (ESF) is added to the construct. Ideal pin sizes for small mammal bone are not known. Intramedullary pins primarily resist bending forces, and the recommended pin diameter should provide sufficient stiffness and strength for the fracture.42,43 Because intramedullary pins do not counteract other forces on fractures—such as rotation, tension, and compression—many of the diaphyseal fractures in small mammals will need additional support. External skeletal fixators, separately or in a tie-in configuration, or external coaptation may be combined with IM pins (Fig. 33-2). Because these are small bones, combining apparatuses may not work as well in small mammals as they do in larger domestic species. In rabbits, fractures occurred from pin insertion of both the IM and ESF pins.2a Therefore choose an intramedullary pin as the fixation method when bending is the major force acting on the bone—for example, in spiral or long oblique fractures. Cerclage wires can be used for spiral and long oblique fractures to help counteract shear forces.
Biodegradable implants are now available that provide adequate mechanical properties yet do not need to be removed and may stress-shield bone less than stainless steel. After implantation, biodegradable intramedullary pins are designed to dissolve into water and carbon dioxide, like polylactides, after a period of time.3,37 These would be excellent features for an implant in small mammals. Some research on these products has been done in a rabbit osteotomy model.44 Although results showed adequate stiffness for healing in this model, clinical fractures in small mammals have both biological and biomechanical features that may affect the outcome. Whether these implants would be stiff enough for clinical use in small mammals is unknown.
Bone Plating
In certain situations, the use of a plate is appropriate for fracture repair in a rabbit or ferret. Because of the conformation of these species, a fracture of the humerus or femur can be difficult to manage with an external fixator. The soft tissue coverage over the femur and humerus is considerable, and external skeletal fixation can be uncomfortable when it is used in this location. If plating is chosen, the implants commonly used are 1.5- and 2.0-mm cuttable plates (Synthes, Ltd., Wayne, PA or Veterinary Instrumentation, Sheffield, UK). These offer the surgeon flexibility in choosing the appropriate length, and the plates can be stacked for added stiffness. The limited-contact dynamic compression plates (LCDCP) or the dynamic compression plates (DCP) that would be long enough to bridge the fracture are too large and stiff for use in these small species. Although DCP and LCDCP plates are made in 2.0-mm size, they are short and therefore may not be the appropriate length for a weight-bearing bone. A recent biomechanical study in rabbit bone had similar results; screw insertion caused cortex fractures. The 6-hole, 2.0-mm DCP in rabbit femurs failed at low loads, usually at the plate or the plate-bone interface. The authors of the study acknowledged that the short plate contributed to the low compressive load in this transverse fracture model.2a The principles of applying AO bone plates are covered in other texts and are beyond the scope of this chapter.25,42
Bone plating in these species presents some specific problems. Clinically, the bones of rabbits have extremely thin cortices, which make screw placement difficult without stripping the cortex. One of the present authors (AK) has experienced complete collapse of the bone while trying to place a screw. In a clinical setting, rabbit fractures are usually comminuted. The blood supply to the bone is already compromised, predisposing the rabbit to osteomyelitis and nonunion. Placement of a bone plate with removal of the remaining periosteum and soft tissues only worsens this problem. Plating in these species often overprotects a fracture, preventing load sharing and causing subsequent delayed healing or nonunion.26,47 If a plate is used, the animal may require staged plate removal, which is costly and unpopular with owners. Another disadvantage is the high cost of equipment and implants.
The new locking compression plate (LCP) (Synthes Vet) uses the concept of external fixation internally near the bone. The stability is from the screw locking into the plate instead of the screw compressing the plate to the bone. Therefore LCPs can be placed without contouring, over the periosteum, and minimally invasively so that rigid fixation is combined with preservation of blood supply to the bone. However, the smallest LCP is 2.0 mm, which may still be too large, depending on the animal. Locking screws have excellent holding power even when placed unicortically, and this may eliminate the complication that was observed in the one recent study on rabbit bone.2a,24,25,30,46 The LCP functions differently than conventional plates and may prove useful in small mammals. To date, there are no reports of the use of LCPs in small mammals.
Like biodegradable pins, biodegradable plates and screws were evaluated in the 1980s in rabbit osteotomy models; however, the perfect balance of a biologically sparing and mechanically stiff implant does not yet exist.37 Biodegradable implants are used clinically only on non-weight-bearing bones.44,45 It is unknown whether these implants will be useful in small mammals.
External Skeletal Fixation
Basic principles for applying external fixators are similar in all species. The pins should be inserted with low rotational speed (150-300 rpm). Manual insertion causes wobble and leads to premature loosening of the pins. High-speed drilling can cause bone necrosis, which also leads to the premature loosening of pins.14,15 Smooth pins have poor holding power and tend to loosen prematurely. Negative-profile threaded pins tend to break at the thread-nonthread interface and are rarely used now that positive-profile pins are available in small sizes.28 Predrilling is the correct method for inserting threaded (positive-profile) pins.28 Imex (IMEX Veterinary Inc., Longview, TX; www.imexvet.com) produces a miniclamp and bar system that can hold positive profile K-wires as small as 0.035 in. (0.9 mm). Many small mammals are too tiny for the connecting bars that are 1⁄8 in. (3.2 mm) in size, but a smaller acrylic or polymethylmethacrylate (PMMA) connecting bar can be used with the Imex miniature INTERFACE pins (IMEX Veterinary, Inc.). The miniature Imex pins are designed as positive-profile pins with a central area that is roughened only to allow acrylic bonding to it. The roughened area is not designed for placement in the bone.
Mechanical studies have tested pin placements and configurations for dogs, and some may apply to small mammals.4 Place the fixator pins approximately 1 cm away from the fracture line. Four pins per bone segment are ideal for maximum stiffness: additional pins increase stiffness insignificantly. However, in these small species, four pins provide too much stiffness and probably will not fit in the fracture segments; therefore we rarely use more than three pins per segment. The fixator pin’s diameter should not exceed 25% to 30% of the bone diameter. Position the connecting bar approximately 0.5 to 1 cm away from the skin. Placement of the bar too far away from the skin decreases the stiffness and strength of the external fixators. Remember to allow for swelling in placing the bars. Spread the pins evenly across the fracture segment to achieve maximum strength. Also remember to place the pins through a separate stab skin incision and not through the fracture opening or original incision.4,15,28 Results of one biomechanical study in rabbits demonstrated that an IM pin-ESF construct can cause fractures on pin insertion and that this construct had less than ½ the compressive and bending strength of normal rabbit bone.2a
Bone healing in rabbits with fractures stabilized by external fixation has been well studied as a research model. The current recommendation for external fixation in dogs and other research models is to stage the removal of the apparatus so that bone is allowed to take over load bearing at an early stage of fixation. This process has been termed dynamization, or staged disassembly.28 In studies of rabbits with tibial osteotomies, the ideal time to remove external fixators was 6 weeks.48 The strength and stiffness of the bone were greater if the fixator was removed at 6 weeks than if it was left in place for 12 weeks.48 Clinically, many factors affect optimal removal time of a fixator, such as the age of the animal, the type of fracture, and the degree of disruption of vascular structures. Therefore the key is to examine these patients every 2 to 3 weeks after surgery and to stage removal when there are indications that the fracture has regained normal stiffness, even though strength may not be normal. Experimentally, removing the fixator at 4 weeks causes some loss of reduction and is probably not advisable, even in perfectly reduced fractures.48
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree