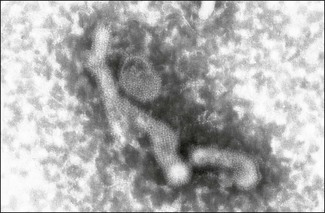
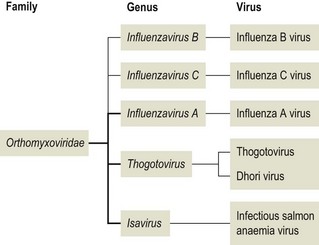
Chapter 60 The name orthomyxovirus is derived from two Greek words, orthos meaning correct or proper and myxa meaning mucus. The family includes the viruses responsible for influenza. Influenza is the Italian form of the Latin word influentia meaning influence because epidemics of infectious disease were believed to result from astrological or occult influences. Orthomyxoviruses are spherical or pleomorhic, enveloped viruses, 80–120 nm in diameter (Fig. 60.1). Extremely long filamentous forms also occur. The envelope is derived from the host cell membrane into which viral glycoproteins and non-glycosylated proteins have been incorporated. The glycoproteins form surface projections, termed ‘spikes’ or peplomers, and are of two types in influenza A and B viruses: a haemagglutinin (H) which is involved in virus attachment and envelope fusion and a neuraminidase (N) which enzymatically destroys viral receptors and promotes both entry and release of virus from infected cells. Influenza viruses haemagglutinate erythrocytes from a wide variety of species. Antibody to the H glycoprotein neutralizes infectivity. The nucleocapsid is helical in symmetry and the genome of linear, negative-sense, single-stranded RNA is segmented into six to eight pieces depending on the genus. Replication occurs in the nucleus of the cell with release by budding from the plasma membrane. Virions are very labile under ordinary environmental conditions, being sensitive to heat, lipid solvents, detergents, irradiation and oxidizing agents. The family consists of five genera: Influenzavirus A, Influenzavirus B, Influenzavirus C, Thogotovirus and Isavirus (Fig. 60.2). Influenza A virus is the most important member of the family and a significant pathogen of animals and man. Influenza B and C viruses are pathogens of man while Thogoto virus and Dhori virus are tick-borne arboviruses isolated from camels, cattle and humans in parts of Africa, Europe and Asia. The genus Isavirus has one member, infectious salmon anaemia virus, which is a cause of disease in Atlantic salmon. Influenza A virus isolates are divided into subtypes on the basis of the H and N antigens. Seventeen H and nine N subtypes have been described to date (Fouchier et al. 2005). New variant influenza A virus isolates emerge at frequent intervals. Two mechanisms are involved in this process, point mutations (antigenic drift) which effect variation within a subtype and genetic reassortment (antigenic shift) which gives rise to novel subtypes. In order to assess the risk posed by the emergence of new variant viruses a precise system of classification of isolates, promoted by the World Health Organization, has been adopted. This system is based on the type, host, geographic origin, strain number, year of isolation and subtype. For example, the prototype equine influenza A virus subtype 1 isolate from 1956 is designated: influenza virus A/equine/Prague/1/56 (H7N7). Isolates with a wide range of H and N combinations have been detected in birds whereas only a few combinations circulate in mammalian species (Table 60.1). Aquatic birds, particularly ducks, are the reservoirs of influenza A virus providing a genetic bank for the generation of novel subtypes capable of infecting mammals. The viruses replicate in the intestinal tract of birds resulting in a faecal–oral transmission pattern. Migratory birds facilitate the dissemination of virus across borders and between continents. Influenza A virus isolates are generally species-specific, but there are many well-documented incidences of transfer between species. In particular, incidences of direct transfer of H7 and H5 subtypes from birds to humans have given rise to concern. Interest has also focused on low pathogenic H9N2 isolates (Lupiani and Reddy 2009). A new pandemic strain of H1N1 appeared in people in Mexico in 2009, spreading rapidly to other parts of the world. The virus contained genetic elements from avian, swine and human isolates. Pigs, the likely source of this virus, are susceptible to infection with this strain. Table 60.1 Influenza A virus infections of veterinary significance The ability of influenza virus to spread in the body is determined by the proteases present in a given tissue and the structure of the haemagglutinin molecule. The production of infectious virions in a tissue is dependent on post-translational cleavage of the precursor haemagglutinin molecule HA0 by host proteases. In the epithelium of the intestinal and respiratory tracts, trypsin and trypsin-like enzymes are capable of cleaving the haemagglutinin molecule of all influenza subtypes. In contrast, protease enzymes present in other tissues are only capable of cleaving the HA0 molecules of virulent subtypes which possess a multiple basic amino acid sequence at the cleavage site. Therefore only virulent subtypes are capable of producing generalized infection characterized by haemorrhages and multifocal necrosis. It is thought that HPAI isolates arise from low-virulence isolates by mutation and that such mutations only take place following the transfer of virus from the natural wild bird host to poultry (Alexander & Capua 2004). • Suitable specimens include tracheal and cloacal swabs, faeces and pooled samples of organs. Suspensions in antibiotic solution are inoculated into nine- to 11-day-old specific pathogen-free or specific antibody-free embryonated hens’ eggs. Allantoic fluid is harvested after four to seven days’ incubation and tested for haemagglutinating activity. • Confirmation of the presence of influenza A virus can be achieved with an immunodiffusion test using a suspension of chorioallantoic membrane from infected eggs and positive antiserum to the nucleocapsid or matrix antigens common to all influenza A viruses. • Commercial antigen-detection immunoassays have been used to detect influenza A viruses in poultry (Slemons & Brugh 1998, Cattoli et al. 2004). The tests are rapid and should detect any influenza A virus as they are generally based on a monoclonal antibody against the conserved nucleoprotein. However, such assays are probably best used as a flock test as they may lack sensitivity. • Isolates may be roughly typed by haemagglutination inhibition or immunodiffusion using broadly reactive antisera. Subtyping is carried out by reference laboratories using monospecific antisera prepared against the 16 haemagglutinin and nine neuraminidase subtypes. • All of the highly pathogenic avian influenza isolates to date have possessed either H5 or H7. However, numerous isolations of low-virulence H5 and H7 subtypes have been made. In order to assess pathogenicity eight to 10 chicks at four to eight weeks of age are inoculated by the intravenous route. Isolates causing more than 75% mortality within eight days (intravenous pathogenicity index of greater than 1.2) are considered highly pathogenic. In addition, genomic sequencing can be used to determine the amino acid sequences at the cleavage site of the haemagglutinin molecule (Wood et al. 1993, Senne et al. 1996). Both HPAI and LPAI isolates of subtypes H5 and H7 are notifiable to the OIE. • Reverse transcription-PCR techniques (Senne et al. 1996, Starick et al. 2000, Munch et al. 2001) as well as real time RT-PCR (Spackman et al. 2002) have been developed for the detection and subtype identification of virus in clinical samples. These methodologies are particularly useful for the rapid detection of subsequent outbreaks once the primary infected premises has been identified and the virus isolate fully characterized. Reverse transcription-PCR based on primers for conserved sequences of the matrix gene has proved useful for screening for all subtypes in samples from a range of different species (Fouchier et al. 2000). Rapid assays for the detection of H5 and H7 virus have also been developed (Slomka et al. 2007) including nucleic acid sequence-based amplification (NASBA) with electro-chemiluminescent detection (Collins et al. 2002, 2003). • Serological testing for antibodies to influenza virus can be done using an agar gel immunodiffusion test, haemagglutination inhibition or competitive ELISA (Shafer et al. 1998). A neuraminidase inhibition test has been developed as part of a strategy to differentiate infected from vaccinated animals (DIVA) (Capua et al. 2003).
Orthomyxoviridae
Host
Antigenic subtypes
Significance of infection
Birds
All subtypes, in most of the possible combinations of the 17 H and 9 N glycoproteins, have been isolated from birds. Highly pathogenic avian influenza (HPAI) isolates restricted to subtypes H5 and H7
Significance varies from inapparent intestinal tract infection to severe generalized infection with high mortality in chickens and turkeys (fowl plague). Wild birds, particularly ducks, act as reservoirs of infection
Pigs
Usually H1N1, H3N2 or H1N2 but other subtypes have also been described
Significant outbreaks of disease typically associated with emergence of new reassortant virus
Horses
H7N7, H3N8. The predominant circulating subtype is H3N8
Acute respiratory disease of young horses
Avian influenza
Pathogenesis
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Orthomyxoviridae
Only gold members can continue reading. Log In or Register to continue