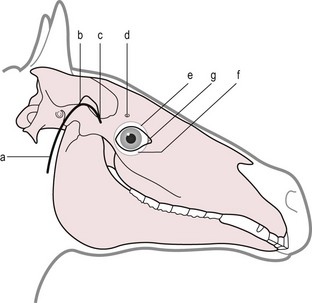
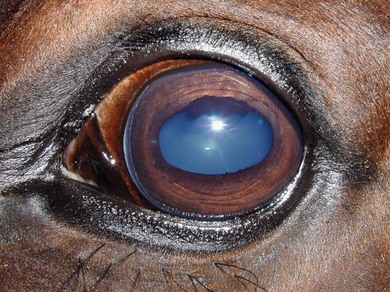
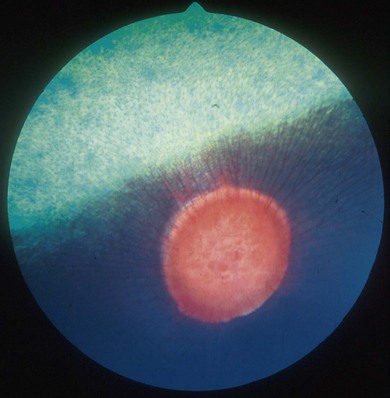
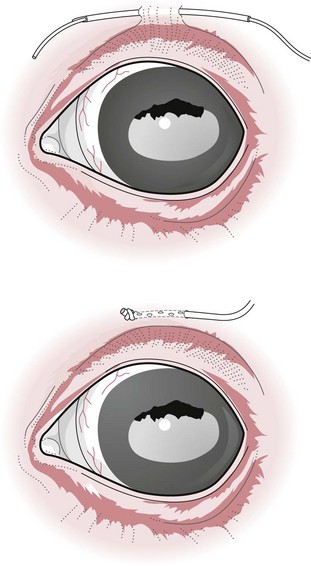
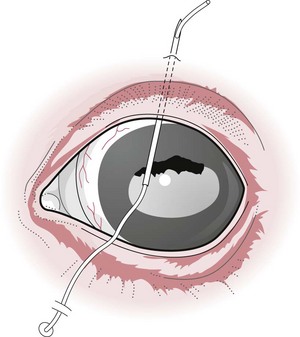
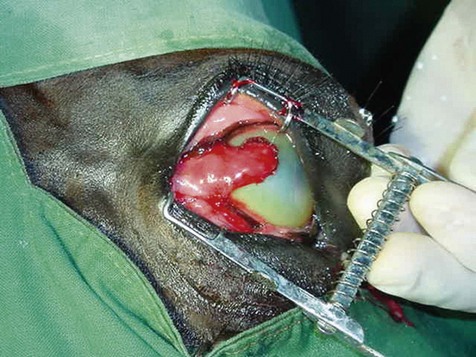
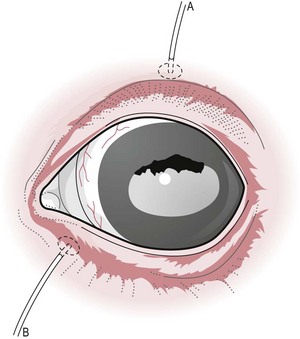
Chapter 12 Disease of the eye, adnexal tissues and/or orbit is common in Equidae due to several factors, including animal behaviour, responsiveness of ocular tissues to injury, and occasionally as a manifestation of systemic disease. Horses are often presented for veterinary examination due to corneal opacity, abnormal eye position, ocular pain, ocular discharge and abnormalities in vision (see section 12.14 for differential diagnoses for common ocular presenting complaints). In addition, examination of the eyes is an integral component of a ‘prior to purchase’ examination. Ocular examination is an important component of a physical examination and requires a systematic approach that can be adapted to the individual case. • Duration of signs and rapidity of onset, i.e. acute, subacute or chronic. • Evidence of ocular pain: lacrimation, blepharospasm, photophobia and avoidance of examination of the affected eye. Pain is usually associated with a lesion in the anterior segment and is seldom a feature of posterior segment involvement. • Evidence of vision loss: inability to avoid inanimate objects, change in temperament, reluctance to ambulate freely in reduced lighting and shying. Acute vision loss is often overt; however, gradual and/or partial loss is difficult to assess. • Known/suspected history of trauma. Trauma may predispose to a variety of conditions including keratitis and uveitis. • Concurrent/previous ocular disease in cohorts and/or related horses. Breed predisposition occurs for several diseases, including cataracts, glaucoma, uveitis, periocular neoplasia and a variety of congenital disorders. A genetic predisposition may exist for some conditions. • Previous treatment and response. • Evidence of systemic disease, e.g. uveitis due to extra-ocular Rhodococcus equi or Streptococcus equi infection, hyphaema secondary to a coagulopathy and ocular involvement in systemic lymphosarcoma. • Otoscope head: a simple method that allows a magnified examination of the external structures of the eye and periocular tissues. • Condensing lens: a +20 to +30 dioptre (D) lens used in combination with a focal light source for indirect ophthalmoscopy permits rapid examination of the fundus. The image obtained is inverted and magnified. Monocular indirect ophthalmoscopes provide an erect and mildly magnified image and a greater field of view than do direct ophthalmoscopes; however, expense precludes widespread availability. Indirect ophthalmoscopy allows the fundus to be surveyed rapidly under low magnification, and any lesions identified can be examined in greater detail by direct ophthalmoscopy. • Direct ophthalmoscopy – close direct ophthalmoscopy – can be used for examination of the adnexa and eye from the cornea to the fundus. The image is real, upright and magnified. Manipulation of the dioptre setting permits focused examination of different ocular tissues without movement of the ophthalmoscope. Distant direct ophthalmoscopy is achieved by selecting 0D, holding the ophthalmoscope at arm’s length and examining the tapetal reflection; any opacity present in the light-conducting tissues of the eye will appear as a silhouette. • Slit lamp biomicroscopy provides superior magnification of external ocular structures, the anterior chamber, iris, lens and anterior vitreous. Determination of the depth of lesions and detection of subtle abnormalities including exudation of protein and/or cells into the anterior chamber are possible. However, equipment is expensive, and experience is required for effective use, limiting the application of this technique in equine practice. • Topical anaesthesia. Desensitization of the cornea and conjunctiva facilitates examination, collection of tissue/fluid samples and cannulation of the nasolacrimal duct. Proxymetacaine hydrochloride or tetracaine hydrocaine solution can be applied by administration directly from a single use ampoule, by spraying the solution using a 1-mL syringe and hub of a 25-gauge needle with the needle removed or by application of an anaesthetic-soaked sterile cotton bud. • Motor and/or sensory nerve blocks (Figure 12.1). Examination in daylight: Important components of examination in daylight/artificial light are: • Orbit and globe. The general appearance of the eyes and adnexa and ocular/facial symmetry is assessed, noting any abnormalities in structure and function of the eyelids (Figure 12.2). The orientation of the cilia (eyelashes) on the superior eyelid should be nearly perpendicular to the corneal surface. Palpation of the orbital rim and supraorbital fossae will assist in detection of disruption of the integrity of the orbit and space-occupying lesions. Eye movement should be assessed bilaterally. In addition, symmetry of pupil size should be assessed because difference in pupil size (anisocoria) reflects a functional (asymmetry of autonomic tone) or structural (e.g. synechia) abnormality. • Neuro-ophthalmic assessment. Ocular disease, disruption of neural pathways associated with vision and/or intra-ocular/extra-ocular muscle dysfunction may result in loss of ocular reflexes/responses that can facilitate localization of a lesion. • Examination of adnexa including the nasolacrimal system. The margins and internal and external surfaces of the superior and inferior eyelids should be examined. The meibomian glands and their openings on the margin of the upper and lower eyelids are visible. Presence and position of the nictitating membrane should be assessed; the palpebral surface can be examined by manual retropulsion of the globe, while examination of the bulbar surface requires application of gentle traction on the free edge with forceps after application of topical anaesthesia. The nasolacrimal drainage apparatus is assessed by confirming the presence of the upper and lower puncta and the nasal ostium. The lacrimal caruncle located at the medial aspect of the palpebral fissue is prominent in the horse. • Ocular surface. Examination of the cornea, conjunctiva and limbus can be performed at this stage to detect alterations in structure. The degree of pigmentation of the conjunctiva is variable. Examination in a darkened area: Once an initial examination in daylight has been completed, a more detailed examination can be performed in a darkened environment such as a stable. • Examination of the cornea by use of focal illumination with/without magnification. Slit-lamp biomicroscopy permits increased acuity of examination, detection of subtle lesions and determination of lesion depth. The normal cornea is translucent, horizontally elongated and slightly wider medially than laterally and devoid of vasculature and pigmentation. The pectinate ligament is appreciated readily as a grey line at the medial (lacrimal) and lateral (temporal) limbus and should not be confused with corneal oedema or cellular infiltration (Figure 12.2). • Examination of the anterior chamber. The depth and clarity of the anterior chamber should be assessed by shining a slit beam of light across the chamber from medial to lateral. Abnormal depth may reflect a change in size or position of the lens or volume of aqueous humor. Opacities indicate the presence of protein (aqueous flare) and/or cells. The ventral aspect of the chamber should be examined for sedimentation of leukocytes (hypopyon) or erythrocytes (hyphaema). • Examination of the iris. The irises of most horses are heavily pigmented; however, pale brown, grey, gold, blue or white coloration may occur and multiple colours may occur within (heterochromia iridis) or between irises. The division between the ciliary and papillary zones of the iris (collarette) is not always distinct, particularly if the iris is heavily pigmented. True albinism is rare; the white/pink iris is frequently very thin and may allow transillumination of the underlying lens equator and ciliary body. The corpora nigra (granula iridica) are heavily pigmented uveal bodies prominent on the dorsal papillary margin and less developed ventrally. The pupil in adults is horizontally ellipse in shape, slightly wider medially, while in foals the pupil is ovoid. Remnants of the embryonic vasculature (persistent pupillary membranes) emanate from the collarette and are common. The iridiocorneal angle can be visualized nasally and temporally. • Examination of the lens. Thorough examination of the lens requires mydriasis achieved by topical application of 1% tropicamide. The lens is examined for abnormalities in clarity, size and position. The normal lens is optically clear; however, in older horses it is yellow, and nuclear sclerosis may be present. Opacities should be described according to size, location and density. Some lens opacities are normal variations (e.g. point of attachment of the hyaloid artery to the posterior capsule (Mitterdorf’s dot), suture lines on the anterior and posterior surfaces and refractile concentric ‘onion rings’). Cataracts are abnormal; however, the degree of vision impairment and likelihood of progression is heavily dependent on cataract type and aetiology. The equator of the lens is not normally visible, and detection indicates lens luxation/subluxation, microphakia (congenital hypoplasia of the lens) or lens coloboma. • Examination of the posterior segment: examination is facilitated by mydriasis and distant and close techniques of direct ophthalmoscopy (vitreous body and fundus), focal light source (vitreous body and fundus), slit-lamp biomicroscopy (anterior vitreous body) and indirect ophthalmoscopy (fundus). The vitreous body is optically clear. The equine fundus comprises a tapetal zone (tapetum lucidum) located dorsal to the optic papilla and a non-tapetal zone. The tapetum is roughly triangular, reflective and often blue/green/yellow in colour. The non-tapetal fundus contains the optic disc and is often heavily pigmented. The optic disc is horizontally ovoid in shape, salmon pink in colour and approximately 40–60 retinal blood vessels radiate from the periphery and extend 1.5–2 disc diameters into the fundus (Figure 12.3). Despite these generalizations, considerable variability exists in the appearance of normal fundus, and differentiating between a normal variant and pathological change can be challenging. • Schirmer tear test (STT). Inadequate tear film production is rare in horses; however, disorders of the preocular tear film (PTF) should be considered when corneal disease of undefined aetiology is present. The Schirmer I tear test is used to assess the aqueous component of the PTF. Stimulated (reflex) tear formation is assessed, and thus the test must be performed prior to application of topical local anaesthetics. A commercially available test strip is bent at the marker notch, and the tip is placed within the conjunctival sac to the level of the bend. The aqueous fraction of the PTF ‘wicks’ along the strip, and the length of wicking from the notch is measured immediately after removal; values of 20–30 mm/min are expected from normal horses while repeatable values of <10 mm/min are consistent with inadequate tear production. • Collection of samples for cytology and microbiology. Samples can be collected from the eyelids, conjunctivae, 3rd eyelid, cornea and adnexal tissues. The method of sample collection is dependent on the lesion. • Conjunctival/palpebral biopsies may be obtained by incisional or excisional techniques. Samples are most appropriate for histopathology, however, they may be submitted for microbiology also. Conjunctival tissue can be collected by sharp dissection after provision of topical anaesthesia. Palpebral lesions can be sampled following local/topical anaesthesia or during general anaesthesia. • Rose Bengal is a red dye used to assess the integrity of the mucin layer of the PTF, as retention of stain indicates staining of epithelial cells in the absence of a mucin covering. In addition, Rose Bengal stains devitalized corneal and conjunctival cells. The dye is an irritant and should be reserved for the detection of subtle defects, including superficial punctate epithelial lesions. • Tonometry. Measurement of intraocular pressure (IOP) is achieved most reliably with a portable tonometer (e.g. Tonopen® XL, Tonovet®). Abolishment of palpebral tone appears to be unnecessary; however, application of topical anaesthesia is required to ensure accurate readings. Sedation may reduce IOP, while a lowered head position may increase IOP. The normal IOP in horses is 23.3 ± 6.9 mmHg (range 7–37). • Nasolacrimal drainage assessment. The upper and lower lacrimal puncta and nasal ostium can be located and inspected for size, position and discharge. Patency can be tested most simply by applying fluorescein stain to the eye; drainage of green stain should be evident at the nasal ostium within 5 minutes. If samples of nasolacrimal discharge are required for bacteriology/cytology or no patency is evident with fluorescein stain, a puncta or the nasal ostium can be cannulated and irrigated after applying local anaesthesia locally. Topical administration of medications is the most common and important method in equine ophthalmology (Table 12.1). However, effective use is influenced by: Table 12.1 Topically administered medications used commonly in Equidae with ocular disease • Corneal/scleral anatomical barriers. Less important for ulcerated cornea or conjunctival disease; however, deep corneal disease (e.g. corneal stromal abscess) and intraocular disease require selection of agents that are able to penetrate the cornea effectively. • Residence time. The majority of aqueous solutions are removed rapidly from the ocular surface by reflex tearing and nasolacrimal drainage. The latter may result in systemic absorption and is of most concern with atropine due to gastrointestinal side-effects. • Frequency of administration. Provided the medication has low tissue toxicity or side-effects, frequent treatment is recommended to maintain therapeutic concentrations. • Volume. Due to reflex tearing and the vertical orientation of the equine cornea, increasing the volume of topical solutions does not improve treatment efficacy, and small volumes (0.1–0.2 mL) are adequate. • Drug vehicle. The formulation influences retention time and available methods of administration. Drugs may be available in solution, suspension or ointment. Ointments generally have the longest retention time and are easiest to instil; however, not all ophthalmic drugs are available in this form. • Drug concentration. The use of fortified solutions can help to maintain therapeutic concentrations at the target site. Techniques for topical application • Solutions/suspensions can be administered easily via a 1-mL syringe with the hub of a 25-gauge needle (with the needle removed); the syringe is steadied by placing the hand on the face of the horse, and the contents are sprayed gently onto the cornea. • Ointments are instilled into the ventral palpebral fornix. • Subpalpebral lavage; commercial (e.g. Mila International) or home-made lavage systems (silastic tubing) provide an effective method of administration of solutions/suspensions, particularly when frequent treatment is required. Placement can be facilitated by sedation of the horse and the use of local motor and sensory anaesthesia or under general anaesthesia. The tubing is placed in the superior or inferior palpebral fornix by use of a stainless steel trocar, ensuring that the tubing does not lie on the cornea (Figures 12.4–12.6). The tubing is attached by plastic guides or glued to the temporal region and then threaded through a series of plaits in the horse’s mane. The free end of the tube should be fitted with a Luer adapter and an injection port and fixed to a plaited piece of mane. The free end should extend to the level of the withers to ensure safety of personnel, as horses with painful ocular disease can be refractory to treatment and exhibit avoidance responses. Figure 12.6 Commercially available subpalpebral lavage devices can be placed in the superior (A) or inferior (B) fornix. • Nasolacrimal lavage using a commercially available nasolacrimal lavage catheter or a canine urinary catheter placed via the nasal ostium and maintained in the nasolacrimal duct. The catheter may be passed through a surgical opening in the false nostril and secured to the adjacent skin. Nasal irritation and traumatic removal of the catheter by the horse and retrograde flow of inflammatory debris onto the cornea are limitations. • Continuous perfusion devices are available and may eliminate the need for frequent manual drug administration. However poly-pharmacy requires drug compatibility to avoid precipitation of drugs and to preserve treatment efficacy. • Contact lenses; therapeutic contact lenses impregnated with medication have received limited attention in horses, however may provide high topical drug concentrations. • Other recent advances in topical drug delivery (e.g. high-viscosity gels, degradable and non-degradable inserts and collagen shields) have yet to be assessed in horses. Solutions and suspensions can be injected under the temporal or dorsal bulbar conjunctiva (Table 12.2). Horses should be sedated and an auriculopalpebral nerve block performed. A cotton bud soaked in local anaesthetic can be applied to the conjunctiva prior to injection. A 25 gauge needle should be used and no more than 1 mL injected. The drug vehicle will influence rapidity and duration of effect; solutions provide rapid and high concentrations in the ocular tissues, while suspensions/emulsions are less potent but have a longer duration of effect. Antibiotics, corticosteroids and atropine can be injected. Solutions for topical use should not be injected; formulations for parenteral use are most appropriate. Occasionally oily solutions can induce granulomatous inflammation. Table 12.2 1. Repair of eyelid lacerations: minimal debridement of wound margins should be performed to preserve the anatomy and function of the eyelids. The eyelids have an excellent blood supply, and even tissue of questionable viability may have the capacity for healing. 2. Nictitans flap. Superficial corneal ulcers and conjunctival grafts can be protected by attaching the 3rd eyelid to the upper eyelid by the placement of 2–4 horizontal mattress sutures. However, the corneal surface is obscured preventing monitoring of the lesion, and the flap does not provide a direct vascular supply to the cornea. 3. Tarsorrhaphy. Surgical apposition of the eyelids can be performed by the use of horizontal mattress sutures placed through the eyelid margin. Indications and limitations are similar to those for a nictitans flap. Partial tarsorrhaphy is useful for improving the retention time of a soft contact lens. 4. Conjunctival pedicle graft. A thin piece of bulbar conjunctiva can be used as a pedicle graft for treatment for deep corneal ulceration, corneal stromal abscess, descemetocoele and corneal perforation (Figure 12.7). Provision of a direct blood supply to the lesion and structural support of the cornea facilitate healing. General anaesthesia of the horse is required. 5. Enucleation may be performed during general anaesthesia of the horse or standing after sedation and use of local blocks, including a retrobulbar block. • Microphthalmos (small and imperfectly formed eye) may be unilateral or bilateral and can be associated with abnormalities of orbital development. Severity varies from subtle microphthalmos and normal eye function to extreme forms where only a small cyst occupies the orbit. While certain breeds (e.g. Thoroughbreds and Clydesdales) appear predisposed, no hereditary basis has been identified. There is no treatment. • Anophthalmos (complete absence of the globe) is rare. • Buphthalmos describes congenital glaucoma with globe enlargement associated with a developmental disorder of the iridocorneal drainage angle (goniodysgenesis). • Strabismus: rarely, deviation of the position of the eye can occur congenitally. • Blunt trauma; retrobulbar/retro-orbital haemorrhage and oedema. Supportive treatment involves systemic anti-inflammatory therapy and prevention of exposure keratopathy (artificial tears/topical ocular lubricating creams). • Cellulitis/abscess (e.g. penetrating trauma, foreign bodies and neoplasia). Orbital pain, blepharoedema, supraorbital fossa swelling, ocular discharge and pyrexia are common. Treatment involves systemic anti-inflammatory and antibiotic therapy, addressing any exposure keratopathy and possibly drainage of a retrobulbar abscess. Prognosis is guarded. • Neoplasia; numerous neoplasms including melanoma, squamous-cell carcinoma, haemangiosarcoma and lymphoma have been associated with exophthalmos of gradual onset. Additionally, strabismus may occur. Ultrasonography, CT and MRI are useful for assessment and can guide diagnostic and surgical procedures. Treatment may be attempted by surgical excision and/or intralesional radiotherapy/chemotherapy; however, the prognosis is often poor to grave. • Hydatid cysts; orbital and retro-orbital (e.g. within paranasal sinuses) localization of hydatid cysts is rare. Due to slow expansion of the cyst, gradual onset of exophthalmos may mimic neoplastic involvement. Ultrasonography may help diagnosis. Surgical evacuation can be attempted and diagnosis confirmed by histopathological examination of the brood capsule.
Ophthalmology
12.1 Examination of the eye and adnexa
History
Ophthalmic equipment and examination techniques
Magnification
Restraint
 an α2-adrenergic agonist; xylazine 0.2–0.5 mg/kg, detomidine; 0.01–0.02 mg/kg, or romifidine 0.04–0.12 mg/kg usually suffices.
an α2-adrenergic agonist; xylazine 0.2–0.5 mg/kg, detomidine; 0.01–0.02 mg/kg, or romifidine 0.04–0.12 mg/kg usually suffices.
Protocol for ophthalmic examination
 Menace response: crude assessment of vision manifested by blinking. An absent menace response may reflect an intra-ocular lesion, damage to visual neural pathways, facial nerve dysfunction, reduced mentation or immaturity (the menace response may not fully develop until 2–3 weeks of age).
Menace response: crude assessment of vision manifested by blinking. An absent menace response may reflect an intra-ocular lesion, damage to visual neural pathways, facial nerve dysfunction, reduced mentation or immaturity (the menace response may not fully develop until 2–3 weeks of age).
 Dazzle reflex results in retraction of the globe and blinking on response to bright light. The response is subcortical and may help to differentiate cortical blindness from disease of the eye, optic nerve or optic tracts.
Dazzle reflex results in retraction of the globe and blinking on response to bright light. The response is subcortical and may help to differentiate cortical blindness from disease of the eye, optic nerve or optic tracts.
 Palpebral and corneal reflexes. Assess the integrity of the trigeminal nerve (sensory), facial nerve and neural connections in the brainstem. Degree and rapidity of eyelid closure after periocular/corneal stimulation are assessed prior to sedation or regional anaesthesia.
Palpebral and corneal reflexes. Assess the integrity of the trigeminal nerve (sensory), facial nerve and neural connections in the brainstem. Degree and rapidity of eyelid closure after periocular/corneal stimulation are assessed prior to sedation or regional anaesthesia.
 Pupillary light reflex (PLR). Assesses the integrity of the retina, subcortical visual pathways, efferent parasympathetic function of the oculomotor nerve (motor) and the iris constrictor muscle. Both the direct and consensual PLR should be assessed (before sedation or use of mydriatics); however, the consensual reflex is weak in the horse. An intact PLR does not indicate vision because the reflex is subcortical.
Pupillary light reflex (PLR). Assesses the integrity of the retina, subcortical visual pathways, efferent parasympathetic function of the oculomotor nerve (motor) and the iris constrictor muscle. Both the direct and consensual PLR should be assessed (before sedation or use of mydriatics); however, the consensual reflex is weak in the horse. An intact PLR does not indicate vision because the reflex is subcortical.
Ancillary diagnostic techniques
 Tissue swabbing and scraping. The conjunctivae and palpebral margins can be sampled without local anaesthesia; however, sampling of the cornea is facilitated by topical anaesthesia. Corneal ulcers can be sampled with a moistened swab or by scraping the ulcer with a Kimura spatula or a scalpel blade handle. Bacteria are most likely to be sampled from the periphery of a lesion, while collection of corneal tissue by deep scraping or partial keratectomy is recommended if mycotic keratitis is suspected. Cytological examination of samples obtained from ulcerative keratitis may reveal leukocytes, and microorganisms, and microbiological culture may identify the causative organism(s). Isolation of microorganisms is not affected by prior use of topical anaesthesia.
Tissue swabbing and scraping. The conjunctivae and palpebral margins can be sampled without local anaesthesia; however, sampling of the cornea is facilitated by topical anaesthesia. Corneal ulcers can be sampled with a moistened swab or by scraping the ulcer with a Kimura spatula or a scalpel blade handle. Bacteria are most likely to be sampled from the periphery of a lesion, while collection of corneal tissue by deep scraping or partial keratectomy is recommended if mycotic keratitis is suspected. Cytological examination of samples obtained from ulcerative keratitis may reveal leukocytes, and microorganisms, and microbiological culture may identify the causative organism(s). Isolation of microorganisms is not affected by prior use of topical anaesthesia.
 Impression smears can by made from lesions of the adnexa by pressing a clean slide directly onto the site of interest. Squamous cell carcinomas and, rarely, other neoplasms, can be diagnosed in this manner.
Impression smears can by made from lesions of the adnexa by pressing a clean slide directly onto the site of interest. Squamous cell carcinomas and, rarely, other neoplasms, can be diagnosed in this manner.
 Fine-needle aspiration of space-occupying lesions involving the external globe and adnexa may harvest cellular tissue suitable for cytological examination. Material aspirated should be transferred immediately to a clean slide.
Fine-needle aspiration of space-occupying lesions involving the external globe and adnexa may harvest cellular tissue suitable for cytological examination. Material aspirated should be transferred immediately to a clean slide.
 Collection of corneal biopsy samples by sharp dissection, preferably during general anaesthesia, for cytology, microbiology and histopathology. Corneal biopsy is best undertaken where suitable facilities and expertise are available.
Collection of corneal biopsy samples by sharp dissection, preferably during general anaesthesia, for cytology, microbiology and histopathology. Corneal biopsy is best undertaken where suitable facilities and expertise are available.
 Fluorescein sodium. This orange dye changes to green in the alkaline environment of the PTF and is used to assess the integrity of the corneal epithelium. The dye does not stain epithelium or Descemet’s membrane (hydrophobic); however, it is rapidly absorbed by exposed stroma (hydrophilic) facilitating diagnosis of ulcerative keratopathies. After application of the dye, the corneal surface should be lavaged with sterile, isotonic saline solution to disseminate the stain and avoid erroneous diagnosis of corneal ulceration. Weak staining, as occurs with corneal microerosions, is best appreciated with cobalt-blue light. Fluorescein can also be used to check for leakage of aqueous humor from the anterior chamber (Seidel test); after application of the dye the corneal surface is examined for the presence of a clear/light green rivulet within the yellow-green fluorescein.
Fluorescein sodium. This orange dye changes to green in the alkaline environment of the PTF and is used to assess the integrity of the corneal epithelium. The dye does not stain epithelium or Descemet’s membrane (hydrophobic); however, it is rapidly absorbed by exposed stroma (hydrophilic) facilitating diagnosis of ulcerative keratopathies. After application of the dye, the corneal surface should be lavaged with sterile, isotonic saline solution to disseminate the stain and avoid erroneous diagnosis of corneal ulceration. Weak staining, as occurs with corneal microerosions, is best appreciated with cobalt-blue light. Fluorescein can also be used to check for leakage of aqueous humor from the anterior chamber (Seidel test); after application of the dye the corneal surface is examined for the presence of a clear/light green rivulet within the yellow-green fluorescein.
 Radiology. Osseous lesions of the orbit and surrounding skull may be identified radiographically; lesions may include fractures, osteomyelitis and neoplasia. Contrast radiography of the nasolacrimal duct (dacrocystorhinography) is useful for assessment of congenital and acquired disorders; the upper lacrimal punctum is cannulated, 5–10 mL of an iodine-based contrast agent is injected, and lateral and oblique radiographs of the head are obtained.
Radiology. Osseous lesions of the orbit and surrounding skull may be identified radiographically; lesions may include fractures, osteomyelitis and neoplasia. Contrast radiography of the nasolacrimal duct (dacrocystorhinography) is useful for assessment of congenital and acquired disorders; the upper lacrimal punctum is cannulated, 5–10 mL of an iodine-based contrast agent is injected, and lateral and oblique radiographs of the head are obtained.
 Ultrasonography is a useful, non-invasive technique for the examination of the globe, adnexa and orbit. Superior image resolution is achieved by use of a high-frequency (7–16 MHz) linear transducer. Indications include any condition that impedes direct examination of part or the entire globe (e.g. blephoedema, diffuse corneal disease, uveitis, cataracts or intra-ocular masses), determination of globe size, assessment of the peri-ocular and retrobulbar soft tissues and examination of the orbit. Identifiable lesions include: structural consequences of uveitis, uveal cysts, cataracts, lens luxation or rupture, vitreal opacities, retinal detachment, enophthalmitis, abnormalities in globe size/shape, intraocular/periocular/retrobulbar masses and orbital fractures.
Ultrasonography is a useful, non-invasive technique for the examination of the globe, adnexa and orbit. Superior image resolution is achieved by use of a high-frequency (7–16 MHz) linear transducer. Indications include any condition that impedes direct examination of part or the entire globe (e.g. blephoedema, diffuse corneal disease, uveitis, cataracts or intra-ocular masses), determination of globe size, assessment of the peri-ocular and retrobulbar soft tissues and examination of the orbit. Identifiable lesions include: structural consequences of uveitis, uveal cysts, cataracts, lens luxation or rupture, vitreal opacities, retinal detachment, enophthalmitis, abnormalities in globe size/shape, intraocular/periocular/retrobulbar masses and orbital fractures.
 CT/MRI. Advanced diagnostic imaging techniques are not used commonly for examination of the eye and adnexa; however, they may permit detection of periocular/retrobulbar masses and osseous lesions.
CT/MRI. Advanced diagnostic imaging techniques are not used commonly for examination of the eye and adnexa; however, they may permit detection of periocular/retrobulbar masses and osseous lesions.
 Nuclear scintigraphy may detect active disease of the bony orbit.
Nuclear scintigraphy may detect active disease of the bony orbit.
12.2 Techniques in ocular therapeutics
Local therapy
Category/drug
Dosage
Comments
Antibiotics
Neomycin /polymyxin B/bacitracin (or gramicidin)
Solution/ointment; q2–6 hr
Broad-spectrum bacteriocidal. Use for superficial ulcers.
Gentamicin
Solution/ointment; q2–6 hr
Bacteriocidal; G– spectrum. Staphylococcus spp.
Tobramycin
0.3% solution; q2–6 hr
Bacteriocidal; G– spectrum, use reserved for severe infections e.g. with Pseudomonas aeruginosa
Chloramphenicol
0.5–1% solution/ointment; q2–6 hr
Bacteriostatic; G+ spectrum
Ciprofloxacin
0.3% solution; q2–6 hr
Bacteriocidal; G– spectrum, use reserved for severe infections by sensitive organisms
Antifungals
Natamycin
5% solution; q1–8 hr
Limited corneal penetration. Good efficacy against filamentous fungi
Amphotericin B
0.15% solution in 5% glucose q2–6 hr
Irritant to cornea. Most active against yeasts
Itraconazole + DMSO
1% with 30% DMSO. q4–6 hr
Excellent corneal penetration. Requires compounding.
Fluconazole
1% solution; q4–8 hr
Fungal isolates from equine keratomycosis often demonstrate poor susceptibility
Miconazole
1% solution; q2–6 hr, 2% cream; q4–6 hr
Good corneal penetration, broad-spectrum and non-irritant. Solution only available from compounding pharmacies. Use vaginal creams only; dermatological creams contain ethyl alcohol that is a corneal irritant
Silver sulfadiazine
1% cream; q4–6 hr
Broad-spectrum
Voriconazole
1% solution; q4–6 hr
Excellent corneal penetration and broad spectrum of fungicidal activity against filamentous fungi
Anticollagenolytics
Potassium EDTA
0.05–1.0% solution; q2–4 hr
Add 1–5 mL of sterile water to a commercial blood collection tube. Potent inhibitor of MMPs
Endogenous serum/plasma
q2–4 hr
Contains several α-globulins that inhibit MMPs and serine proteases. Samples should be kept refrigerated and sterile and replaced every 5 days.
Tetanus anti-toxin
q2–4 hr
Commercial source of α-globulins
Doxycycline
0.1–0.3%; q4–6 hr
Inhibitor of MMP function. Must be compounded.
Acetylcysteine
5–10% solution; q1–4 hr
Inhibitor of MMP function. Mucolytic action may compromise the tear film with prolonged use.
Mydriatics
Atropine
1% solution; to effect
Potent mydriatic and cycloplegic. Frequent use may predispose to reduced faecal output and/or colic.
Tropicamide
1% solution; once
Short-acting mydriatic used to facilitate examination of lens and posterior segment
Phenylephrine HCl
10% solution
May be combined with atropine to improve mydriasis in refractory cases
Anti-inflammatories
Prednisolone acetate
1% solution; q1–6 hr
Potent corticosteroid with excellent ocular penetration. Immunosuppressive and delays healing.
Dexamethasone HCl
0.05–0.1% solution; q1–6 hr
Potent corticosteroid with excellent ocular penetration. Immunosuppressive and delays healing.
Flurbiprofen
0.03% solution; q1–6 hr
NSAID, good ocular penetration. Delays corneal epithelialization
Cyclosporine A
0.2% ointment; q6–12 hr
Potent inhibitor of T-cell function. Poor corneal penetration and weak direct anti-inflammatory effect.
Suprofen
1% solution; q4–6 hr
NSAID. Good ocular penetration. Low concentrations do not affect epithelial migration.
Subconjunctival injection
Medication
Concentration for topical use
Dosage for subconjunctival injection
Cephalothin
65 mg/mL
Cephazolin
33–50 mg/mL
100 mg
Ticarcillin
6 mg/mL
100 mg
Penicillin G
1 × 106 IU/mL
0.5 × 106 IU/mL
Gentamicin
10–15 mg/mL
20 mg
Amikacin
6.7–15 mg/mL
25–75 mg
Tobramycin
15 mg/mL
20–40 mg
Chloramphenicol
5–10 mg/mL
50–100 mg
Atropine
1–2 mg
Triamcinolone acetate
1–2 mg q7–10 days
Methylprednisolone acetate
20 mg q3–4 days
Surgery
12.3 Globe and orbit
Acquired abnormalities
Exophthalmos