CHAPTER 29 Ophthalmology
Ophthalmic Examination and Diagnostic Techniques
Examination Techniques and Order
The examiner should begin at eye level with the cat. The cat’s head should first be observed from a distance, avoiding excessive manipulation of the face by the restrainer. This allows for detection of nonocular abnormalities that may be related to the ocular disease, such as facial asymmetry, oral or nasal discharge, and the presence of a head tilt. The examiner should then perform the neuro-ophthalmic examination (Table 29-1). The neuro-ophthalmic assessment for cats can yield very different results than that for the dog. For example, the menace response (Figure 29-1) tends to be inconsistently elicited in cats, with many normal cats failing to blink in response to a menacing gesture. Likewise, stressed cats with higher sympathetic tone often have resting mydriasis and diminished pupillary light reflexes (PLRs).
TABLE 29-1 Components of the Neuro-Ophthalmic Examination
| Test | Tested Structures |
|---|---|
| Menace response | CN II and VII, visual cortex, and cerebellum |
| Palpebral reflex | CN V and VII |
| Oculocephalic reflex | CN III, IV, and VI |
| Direct and consensual pupillary light reflexes | CN II and III and central visual pathways excluding the visual cortex |
| Dazzle reflex | CN II and VII and subcortical visual pathways |
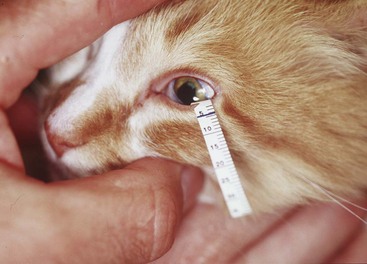
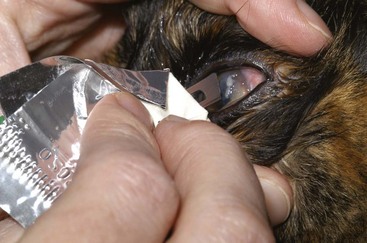
If a STT is to be performed, it must be done before any eye drops are applied to the eye. It is performed in the same manner as in the dog (Figure 29-2); however, normal STT measurements vary widely in cats.194 For example, the authors have recorded STT values less than 5 mm/min in cats without detectable ocular disease. Conversely, cats with significant keratoconjunctival disease may have STT results within the normal reference range.106 Such discrepancies emphasize the importance of interpreting the STT in context with the overall clinical examination and comparing them between the affected and unaffected eyes in cats with unilateral or asymmetric ocular disease. After the STT, intraocular pressure (IOP) should be assessed. Although there is some variability, normal feline IOP tends to be between 10 and 25 mm Hg.140 Both the Schiotz and TonoPen tonometers require application of a topical anesthetic agent before obtaining an IOP measurement, whereas the TonoVet does not (Figure 29-3). Although retropulsion can be useful in detecting space-occupying lesions of the orbit, it is not considered an acceptable technique for measurement of IOP. If the IOP is within normal limits, a single drop of 0.5% or 1% tropicamide should then be applied to each eye to achieve pupillary dilation, which is essential for examination of the lens, vitreous, and ocular fundus. In cats tropicamide induces mydriasis within 15 minutes, for 8 to 9 hours.87 Pharmacologic mydriasis is not recommended if the IOP is elevated because dilation may further increase the IOP.
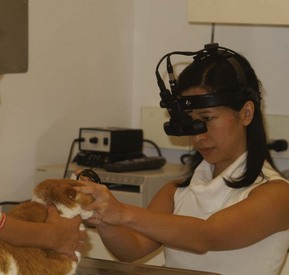
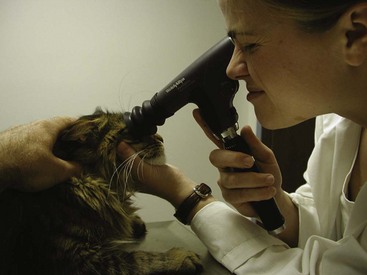
The remainder of the ophthalmic examination may be performed during and after pupillary dilation, and the techniques used in cats differ little from those used in other species. Sequential examination of all structures moving from peripheral to axial and from superficial to deep ensures a complete and orderly examination. The examiner should begin with retroillumination of the tapetal reflection from arm’s length (Figure 29-4) to identify opacities in the visual axis. Magnification, in the form of head loupes (Figure 29-5), should then be employed throughout the entire ophthalmic examination, except for the fundic examination. The examiner should employ focal illumination and transillumination (Figure 29-6) from various angles when assessing the ocular surface. Depth and spatial relationships are best assessed using the slit beam of the direct ophthalmoscope. The detection of aqueous flare (plasma protein in the anterior chamber) is a pathognomonic change in anterior uveitis, and therefore its detection forms a critical part of the ophthalmic examination in all cats. Aqueous flare is best detected when the room has been darkened completely and the smallest, most focal white light beam on the direct ophthalmoscope head is used very close to the cat’s eye to examine the clarity of the anterior chamber. Any “smokiness” detected as the beam traverses the anterior chamber between the cornea and the lens should prompt consideration of and further investigation for anterior uveitis. The iris face is a frequent site of pathology in cats and must be evaluated before dilation. By contrast, thorough assessment of the lens, vitreous, and fundus can be performed only after full dilation has been achieved. Also, aqueous flare may be more easily detected after full mydriasis has been achieved, insofar as the black background of the pupil provides contrast with which to view the gray beam of light traversing the anterior chamber. Direct and indirect ophthalmoscopy are the two traditional methods for examining the fundus (Figure 29-7). Direct ophthalmoscopy is relatively easy to learn and provides the examiner with an upright image of the retina but is not a good method for examination of the fundus. The main disadvantage of direct ophthalmoscopy is the limited field of view, a result of extreme magnification. This makes complete retinal examination difficult, and peripheral regions and smaller lesions are often missed. Indirect ophthalmoscopy is considered to be the best method for viewing the fundus, despite the steeper learning curve. The image produced is an inverted representation of the fundus. When the binocular headset (Figure 29-8) is used, indirect ophthalmoscopy provides superior depth perception. The larger field of view obtained by indirect ophthalmoscopy makes complete fundic examination easier and allows the practitioner to view a larger portion of the fundus in less time, which is extremely valuable for uncooperative patients. The direct ophthalmoscope can then be used for more detailed examination of any focal lesions identified. A newer ophthalmoscope, the Panoptic offers a field of view and level of magnification intermediate to the traditional ophthalmoscopes (Figure 29-9). Although it is unable to provide much depth perception, its ease of use and moderate field of view make the Panoptic a reasonable compromise between direct and indirect ophthalmoscopy.
The Jones test is an assessment of nasolacrimal duct patency (Figure 29-10). A minimum of 2 minutes after placing fluorescein dye into the conjunctival sacs, the nares and mouth are examined for evidence of fluorescein. Presence of fluorescein (positive Jones test) confirms patency of the nasolacrimal duct, whereas absence of fluorescein (negative Jones test) is suggestive of obstruction and should be followed with nasolacrimal flushing.
The tear film break-up time (TFBUT) evaluates stability of the precorneal tear film. It is the time between eyelid opening and the first spot of evaporation within the precorneal tear film. The examiner first places fluorescein dye into the conjunctival sac, then closes the eyelids. Timing begins when the eyelids are opened. Using magnification, the examiner observes one area of the cornea, usually the dorsolateral aspect. Timing ends when the first black spot, signifying evaporation, appears within the green tear film. The normal TFBUT in a cat is 16.7+/−4.5 seconds,36 whereas more rapid TFBUTs suggest tear film instability.
Ancillary Tests
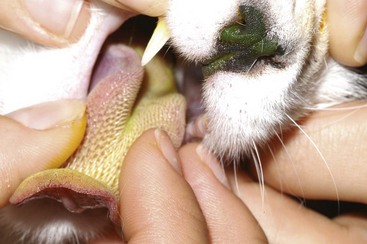
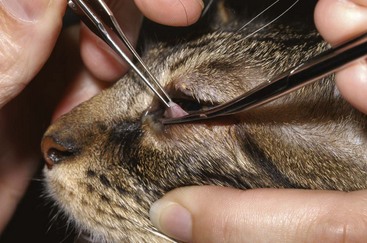
Techniques for ancillary diagnostic tests such as corneal scrapings (Figure 29-11), conjunctival swabs, and conjunctival biopsies (Figure 29-12) are identical to those for the dog. However, the effect of topical anesthetics required to obtain these samples is shorter in cats than it is in dogs. Whereas a single drop of 0.5% proparacaine provides up to 45 minutes of corneal anesthesia in the dog, the same dose achieves only 25 minutes of corneal anesthesia for the cat, with maximal effect 5 minutes after application.16,88 This time difference should be taken into account if the practitioner is unable to obtain samples shortly after administering topical anesthesia. Cannulation of the nasolacrimal puncta is more challenging in cats than dogs because of the tight fit of the eyelids to the globe. Also, in cats more often than in dogs, the nasal opening of the nasolacrimal duct is located more caudally, such that fluid will be flushed into the oral cavity rather than out the nares (as with fluorescein in Figure 29-10).
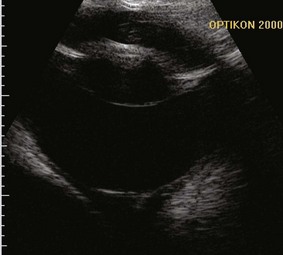
Advanced imaging is required when opaque ocular media prevent complete examination of the globe or when orbital or neurologic disease is suspected. Ocular ultrasound is very useful for characterizing lesions within the globe but may not allow full evaluation of orbital lesions156 (Figure 29-13). Skull radiography and computed tomography (CT) also may not clearly show borders of a lesion, but they will detect bony changes.156 Magnetic resonance imaging (MRI) is of limited value for bony lesions but offers excellent resolution of soft tissues, including the globe, orbit, and optic nerves.73
Orbital Disease
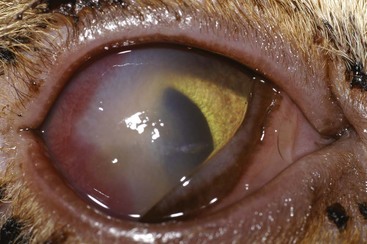
In cats the most common clinical sign accompanying orbital disease is exophthalmos.66 Epiphora, enophthalmos, strabismus, elevation of the third eyelid, and decreased retropulsion are other possible examination findings (Figure 29-14). If eyelid movement has been compromised, exposure keratitis or corneal ulceration may also be found (Figure 29-15). Inflammatory and neoplastic conditions make up the bulk of feline orbital disease; orbital vascular anomalies and cystic lesions have not been reported in cats. Identification of the underlying disease process is important because of significant differences in treatment and prognosis. At minimum, a complete blood count (CBC), serum biochemical profile, urinalysis, and fine-needle aspirate or biopsy of the orbital lesion are recommended. Based on the results of these tests, CT or MRI may be warranted to determine the extent of the lesion.
Infectious and Neoplastic Orbital Disease
Orbital cellulitis and abscess formation in the cat are diagnosed and treated in a similar manner as in dogs. As in dogs, orbital extension of dental disease is responsible for many of these cases, but foreign body migration, fungal infections, and iatrogenic trauma (mainly during dental procedures) are also documented causes.110,157 However, these conditions occur much less frequently in cats than in dogs, and neoplasia should always be considered when a cat presents with orbital disease.157 In cats the majority of orbital neoplasia is secondary, with direct extension from adjacent structures accounting for 71% of cases.66 Squamous cell carcinoma (SCC) is the most common orbital neoplastic process in cats,66 but many other neoplasms may occur.* Regardless of type, 90% of orbital neoplasms are malignant, with mean postdiagnosis survival times less than 2 months.8,66
Ocular Proptosis
Ocular proptosis is seen less commonly in cats than in dogs, likely because of cats’ deep orbits and relatively tightly fitting adnexa. For this reason the amount of force required to displace the globe from the feline orbit is large, and cats with ocular proptosis usually present with severe intraocular trauma, as well as concurrent cranial or systemic injuries that require more immediate attention. Ocular proptosis in cats often occurs in conjunction with skull fractures and trauma to the globe.65 As with dogs, enucleation or emergency replacement of the globe followed by temporary tarsorrhaphy is required. However, the prognosis after traumatic ocular proptosis in cats is worse than in dogs. In one retrospective review, all proptosed feline eyes were permanently blind, and 12 of 18 required enucleation.65
Feline Orbital Pseudotumor
Feline orbital pseudotumor (FOP) is currently described as a progressive inflammatory disease with similarities to idiopathic orbital inflammation in humans. However, only eight feline cases have been described, and the definition is still evolving.15,127 All affected cats were middle-aged to older.15,127 The majority of cats presented with unilateral disease that later became bilateral. In all cases onset of clinical signs was insidious, ultimately characterized by exophthalmos, lagophthalmos, exposure keratitis, and restriction of ocular movements.15,127 Resistance to retropulsion was also found in the majority of cases.15,127
No specific changes are seen on blood work, and fine-needle aspirates tend to be nondiagnostic. Orbital ultrasound may show increased echogenicity of orbital tissues.15 CT appears to be the most useful imaging modality, often revealing physical compression of the globe. Biopsy of orbital tissues (often obtained during enucleation or exenteration) is essential, with histopathology being characterized by proliferating fibrous tissue with lymphocytic–plasmacytic inflammation.15
As for its human counterpart,20 prognosis for FOP is poor and specific therapeutic guidelines do not exist. Immunosuppressive doses of systemic corticosteroids, oral antibiotics, and radiation therapy have all been attempted with little success.15,127,192 One author reports having managed FOP for a period of 1.5 years with immunosuppressive corticosteroid therapy before clinical disease returned.192 This author also reports clinical improvement in one cat after radiation therapy, but this case has not been described nor followed up in the literature.192 Enucleation or exenteration followed medical therapy in all published cases,15,127 and in one case series, all seven cats were eventually euthanized because of either recurrence of disease or appearance of the disease in the contralateral orbit.15
A viral etiology has been suggested for FOP, perhaps in part because of the suggestion that herpes simplex virus (HSV-1) is a proposed cause of human idiopathic orbital inflammation.189 In the case series of seven cats, three exhibited signs suggestive of feline herpesvirus (FHV-1) infection, including upper respiratory disease before or after development of clinical signs of orbital disease, followed by development of gingival hyperplasia.15 However, the authors were unable to establish FHV-1 infection in these cats. More recently, FOP has been suggested to be a neoplastic disease, with spindle cell sarcoma identified in the orbital tissue of one cat and the suggestion that FOP be renamed feline restrictive orbital sarcoma.48
Eyelid and Adnexal Disease
Eyelid Agenesis
Eyelid agenesis, or eyelid coloboma, is the most common congenital eyelid disease of cats.133 It has been documented to occur in the Persian, Burmese, and domestic shorthair, as well as snow leopards and a Texas cougar.11,40,100,124,205 Affected cats have bilateral, incomplete development of the upper lateral eyelids (Figures 29-16 and 29-17). The extent of the defect ranges widely, from a barely perceptible absence of the eyelid margin at the lateral extent of the eyelid to complete absence of the upper eyelid margin. The cause is unknown; viral etiologies, intrauterine events, and genetic mutations are proposed mechanisms, although there is no clear evidence supporting any of these theories.124
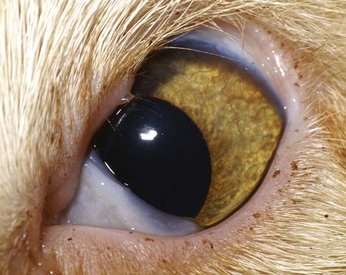
FIGURE 29-17 Agenesis of almost 50% of the left upper eyelid. Although the eyelid defect in this patient is larger than the defect in Figure 29-16, the degree of keratitis is less.
(Image courtesy UC Davis Veterinary Ophthalmology Service.)
Most owners do not notice abnormalities until kittens are a few months of age, probably because of the small eye size of young kittens. On occasion, eyelid agenesis has been mistaken for upper eyelid entropion; however, close inspection reveals absence of the eyelid margin rather than an inward roll. Varying degrees of chronic keratitis resulting from trichiasis and lagophthalmos almost always accompany the eyelid defect (see Figure 29-16). The conjunctival fornix at the site of the malformed eyelid also tends to be shallow or replaced by a thin band of conjunctiva directly connecting the globe to the eyelid. Other ocular abnormalities may accompany eyelid agenesis—most commonly, persistent pupillary membranes, which are remnants of dysplastic uveal tissue visible as thin sheets of pigmented tissue usually extending from the iris to the cornea. Colobomas of the iris, choroid, or optic nerve; retinal dysplasia; and keratoconjunctivitis sicca may also accompany eyelid agenesis.71,124 Like the eyelid defect itself, the associated developmental abnormalities range in severity, sometimes even among kittens from the same litter.124
Treatment varies with the extent of the eyelid malformation. Very small defects may require cryoepilation or primary closure for resolution of trichiasis; however, most defects of clinical significance require more extensive blepharoplasty. Surgical reconstruction usually involves two separate surgeries, the first to correct the defect and the second to correct residual trichiasis. The most common procedure is rotation of a flap of skin from the lower eyelid with a functional outcome (Figure 29-18). Subsequent cryoepilation or a Holtz–Celsus procedure can be employed to address trichiasis from the skin flap.
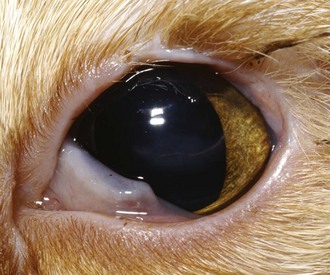
FIGURE 29-18 Patient in Figure 29-17 after completion of a rotating skin flap and subsequent cryoepilation of resulting trichiasis. Although the eyelids are not completely normal, there is improved coverage of the globe during normal blinking and the trichiasis is decreased from before surgery.
(Image courtesy UC Davis Veterinary Ophthalmology Service.)
Entropion
In the authors’ experience, feline breed-related entropion differs greatly from the same condition in dogs. In cats the majority of breed-related entropion is mild and located at the medial aspect of the lower eyelid in brachycephalic cats (Figure 29-19). The clinical significance is often minimal but may be associated with epiphora and tear staining caused by wicking of the tears by trichiasis, kinking of the nasolacrimal canaliculi, and frictional irritation of the cornea. However, these cats frequently have other abnormalities, such as exophthalmos, poor corneal sensitivity, unstable tear film, low blink rates, and lagophthalmos, that predispose them to chronic keratitis, including sequestrum formation. In these patients medial canthoplasty may be necessary to correct the entropion, lagophthalmos, and macropalpebral fissure. Breed-related entropion is believed to be more clinically significant in intact male Maine Coon cats, where it may be related to excessive skin around the face.202
Unlike breed-related entropion, acquired entropion in cats is often associated with clinically significant keratitis. Acquired entropion occurs in response to a primary pathologic process, typically as a result of blepharospasm or symblepharon formation caused by keratoconjunctivitis, or because of changes in globe position or size owing to enophthalmos or phthisis–microphthalmos, respectively. Enophthalmos may be seen in older cats or cachexic cats because of the loss of orbital fat. Surgical correction of feline entropion uses similar techniques as for the dog, which are published elsewhere. However, treatment (and preferably resolution) of any underlying cause before surgery is essential, and some authors have suggested that although the surgical technique is the same, cats require excision of a larger amount of tissue than do dogs.202
Herpetic Dermatitis
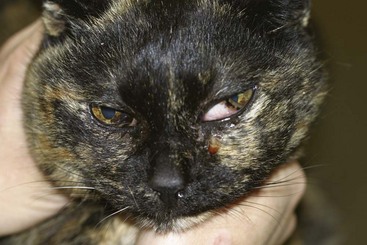
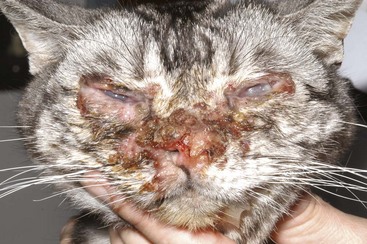
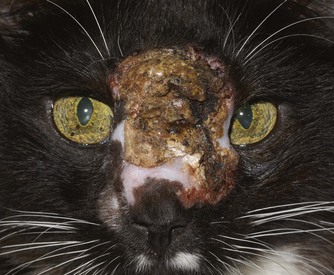
Periodically, FHV-1 has been identified as a cause of dermatologic lesions, particularly those surrounding the eyes and involving nasal skin of domestic and wild felidae* (Figures 29-20 and 29-21). This is not surprising given the marked epithelial tropism of this virus and the reliability with which HSV-1 causes dermal lesions.115 Herpetic dermatitis typically presents with raised, thickened plaques and chronic, nonhealing cutaneous ulcers. Most commonly, the periocular skin, nasal planum, and the skin around the muzzle are affected; however, lesions may also occur on forelimbs and other sites in contact with oral and ocular secretions.80,97 Oral ulcers and rhinitis may accompany dermal lesions.80,132,184 Most published cases had concurrent immune compromise, in the form of recent glucocorticoid administration or systemic disease.80,184 Histologic changes are often diagnostic; however, because of the eosinophilic infiltration, misinterpretation of lesions as eosinophilic granuloma may occur.80 Unlike herpetic corneal or conjunctival disease, which is not reliably diagnosed using the polymerase chain reaction (PCR), this assay seems diagnostically useful for herpetic dermatitis. When results of histologic examination were used as the gold standard in one study of cats with dermatitis, sensitivity and specificity of the PCR assay were 100% and 95%, respectively.93 Although prospective controlled studies are lacking, this disease seems responsive to systemic administration of famciclovir (see the section on corneoconjunctival disease later in this chapter).
Eyelid Neoplasia
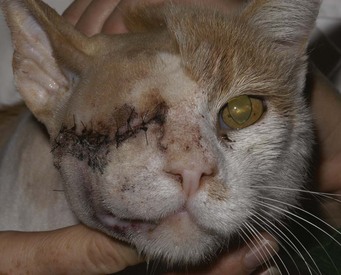
In contrast to dogs, most feline eyelid neoplasms are malignant, and many tumors are locally invasive.125,138,204 SCC is the most common feline eyelid tumor, although a variety of neoplasms have been reported (Figure 29-22).* Eyelid neoplasia tends to occur in cats older than 10 years of age.125,138 However, one study found SCC affected slightly older cats (12.4 years), whereas mast cell tumors were more common in younger cats (6.5 years).138 This same study also underscored the systemic significance of eyelid tumors. Cats with eyelid or third eyelid lymphoma, adenocarcinoma, SCC, and peripheral nerve sheath tumors frequently experienced tumor recurrence or died as a result of tumor-related causes.138 Conversely, the literature suggests that recurrence is unlikely after excision of hemangiosarcoma or mast cell tumor.83,138,149 Regardless, presurgical diagnosis through incisional biopsy or aspirate, diagnostic assessment for systemic metastases, and tumor resection with wide surgical margins are recommended for all eyelid tumors of cats. Given the essential role that eyelids play in ocular, especially corneoconjunctival, health, extensive blepharoplasty procedures are often required to guarantee postoperative eyelid function when surgical margins result in loss of more than 25% of the eyelid length (Figure 29-23). In some cases sufficiently wide margins cannot be achieved without enucleation or exenteration of a normal globe, and even then these procedures are often accompanied by extensive axial pattern flaps to cover the defect created (Figure 29-24).
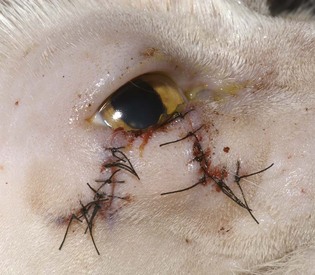
FIGURE 29-23 Immediate postoperative photograph of the cat in Figure 29-22. An advancement flap was performed to close the large defect created by excision of the eyelid tumor with adequate surgical margins.
(Image courtesy UC Davis Veterinary Ophthalmology Service.)
Apocrine hidrocystoma is an unusual and relatively uncommon neoplasm affecting feline eyelids and, as one of the few benign neoplasms affecting the eyelid of cats, warrants separate discussion. It represents a proliferative lesion of the apocrine glands within the eyelid. Although histologic confirmation is necessary for definitive diagnosis, the typical appearance of the lesion is a smooth-surfaced, hairless, often pigmented, slightly translucent eyelid mass (Figure 29-25). Apocrine hidrocystomas may occur singly, or there may be multiple lesions on the same eyelid.69 Persian cats are predisposed.25,69,208 Isolated masses may be removed with simple excision, although recurrence is common and cryosurgery is recommended as an adjunctive therapy.25,28 There is one report of ablation using trichloroacetic acid (TCA).208 In this case the hidrocystomas were eliminated without adverse effects, and no recurrence was noted 1 year after treatment.208 This treatment should be used with caution because TCA has the ability to cause painful blisters and burns to normal skin.208
Third Eyelid Disease
Disease affecting only the third eyelid in cats appears to be uncommon; however, the third eyelid is occasionally the site of primary neoplasia.138,149 Prolapse of the gland of the third eyelid (“cherry eye”) has been reported in the cat, and it has been suggested that the Burmese may be predisposed.100 As in the dog, surgical replacement of the gland is required, with specific techniques being published elsewhere.7 Elevation of the third eyelid, without gland prolapse, may be a sign of Horner’s syndrome if accompanied by signs such as miosis and ptosis. Pharmacologic testing and other diagnostic investigations are similar to those used for dogs. Cats also occasionally have bilateral elevation of the third eyelids without other ocular abnormalities (Haw’s syndrome) (Figure 29-26). Infectious etiologies have been proposed for this syndrome when it occurs in conjunction with diarrhea,123,131 but the condition is thought to be self-limiting when no other abnormalities are found.
Nasolacrimal System Disease
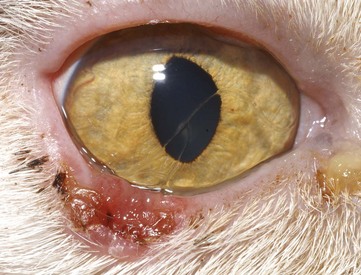
Primary disease of the nasolacrimal duct occurs infrequently in the cat. However, its physical characteristics make it prone to involvement when adjacent structures are diseased. For example, the lack of osseous protection of the distal lacrimal sac leaves it susceptible to inflammation from adjacent respiratory mucosa.139 In addition, a portion of the nasolacrimal duct lies in very close proximity to the root of the canine tooth, which is a common site of dental disease in cats.122,139 Because of ventromedial entropion, the puncta are often in poor apposition with the globes of brachycephalic cats. This, combined with the fact that the nasolacrimal ducts of brachycephalic cats undergo sharper turns, increases the chances of impaired tear drainage and epiphora21 (Figure 29-27).
Corneal and Conjunctival Disease
Feline Herpesvirus
As a typical alphaherpesvirus, FHV-1 is highly host-specific; replicates rapidly in epithelial cells, where it causes cytolysis; establishes lifelong latency within ganglia; periodically reactivates from latency, especially during periods of stress; and during reactivation can either cause cell lysis again or activate immune-mediated pathology. Although the virus typically persists for life in the ganglia of latently infected cats, it is extremely labile in the environment and is susceptible to most disinfectants and to desiccation. For example, FHV-1 is relatively rapidly killed in fluorescein stain and proparacaine; however, it can survive in eyewash for 5 days.181 Assuming that adequate hygiene is practiced in veterinary clinics, this short environmental survival time means that cats, rather than fomites, are the major source of viral persistence. Infection results from direct mucosal (oral, nasal, conjunctival) transfer of viral-laden macrodroplets, generated during sneezing but not normal respiratory movements. Up to 97% of cats are seropositive, and the virus is considered to be responsible for 45% of all upper respiratory infections.58,118,182 All studies to date suggest little variation in pathogenicity between viral strains, found worldwide.95,111–112,166
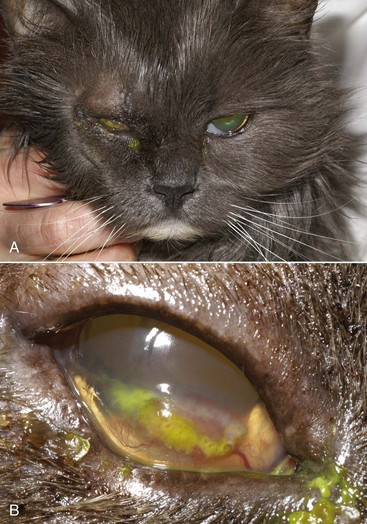
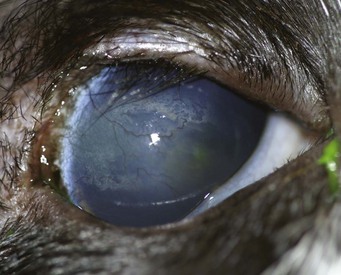
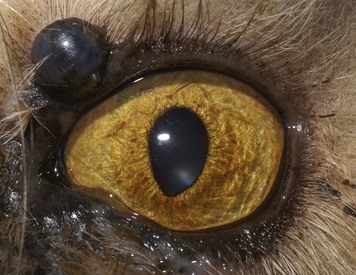
After initial infection of a naïve cat, the incubation period is 2 to 10 days; however, this and disease severity are likely dose dependent. Viral shedding in ocular, oropharyngeal, and nasal secretions begins as early as 24 hours after inoculation and can persist for 1 to 3 weeks. Subsequent intermittent shedding is characteristic of the lifelong carrier state. The virus causes disease through a number of theorized mechanisms, each of which requires a different therapeutic approach. The initial period of rapid intraepithelial replication and associated cytolysis manifests clinically as erosion and ulceration of the ocular, oropharyngeal, and nasal mucosa, often with a serosanguineous discharge (Figure 29-28). The pathognomonic dendritic ulcerative pattern is sometimes seen in the cornea (Figure 29-29); however, their absence should not be used to rule out FHV-1 as a causative agent.
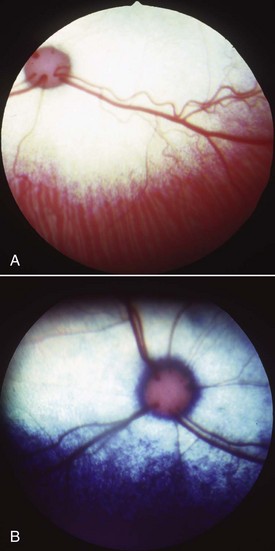
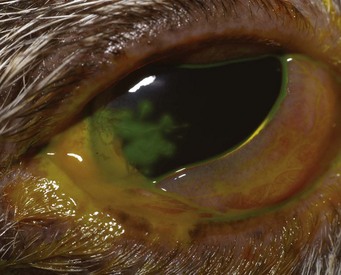
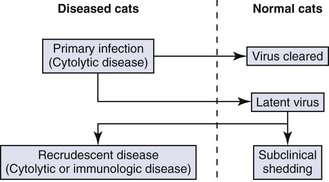
Primary disease is usually self-limiting within 10 to 20 days. Viremia occurs during this phase of infection, but because systemic FHV-1-related syndromes are poorly defined, its significance is unknown. Also during this period, viral latency is established in the majority of cats, often as early as 4 days after infection, presumably by way of the ascent of the sensory axons of the trigeminal nerve. Latency is a period of viral quiescence during which there is no clinical evidence of disease, no histologically detectable inflammation within nerves or ganglia, no detectable virus using standard culture techniques, and limited viral transcriptional activity. In some animals intermittent episodes of viral reactivation from the latent state may be followed by centrifugal spread of virus along the sensory axons to peripheral epithelia. Viral recrudescence occurs when this results in clinically evident disease at peripheral epithelial sites. Recrudescent disease may be of the same ulcerative character as primary disease or may cause disease through recruitment of host immunologic mechanisms. It is often unilateral and typically not associated with generalized malaise or severe respiratory signs. The severity and extent of recrudescent disease range widely among individuals and even between episodes, making diagnosis more challenging. However, as a rule, conjunctivitis is milder and less ulcerative than seen in the primary infection, sometimes instead with substantial conjunctival thickening and hyperemia secondary to inflammatory cell infiltration. Corneal recrudescence may involve only the epithelial tissues, in which case dendritic and later geographic corneal ulceration may be seen; however, stromal keratitis is also common. This is characterized by vascularization, fibrosis, edema, and white blood cell infiltration of a nonulcerated cornea (Figure 29-30) and is believed to be due to FHV-1 antigen persistence within these tissues. Predicting which animals within a population are going to suffer recrudescent disease is currently not possible. The important concept is that although the majority of cats appear to become latently infected, only a minor percentage of them experience chronic or recurrent herpetic disease (Box 29-1). The pathogenesis of FHV-1 is summarized in Figure 29-31.
BOX 29-1 Important Virologic Considerations for Cats Infected with Feline Herpesvirus-1
1 Feline populations themselves, not the environment, act as the major reservoir of feline herpesvirus-1 (FHV-1).
2 FHV-1 infection (but not necessarily disease) is common in individual cats.
3 FHV-1 establishes lifelong latency within neural tissues in approximately 80% of infected individuals.
4 FHV-1 reactivates from latency commonly with or without obvious cause and with or without clinical evidence of reactivation.
5 FHV-1 reactivation is associated with recrudescent disease in only a small percentage of cats.
6 FHV-1 induces disease by way of (at least) two distinct mechanisms, with important therapeutic implications:
Chlamydophila felis
Although C. felis has some unique biological features, it shares a number of them with FHV-1: It is an obligate intracellular organism spread by macrodroplets and direct contact, it replicates within epithelial cells, it persists within the host but poorly in the environment, and it is highly host adapted. The recent reclassification of the feline chlamydial organism from Chlamydia psittaci var. felis to its own distinct species reflects this host specificity and low zoonotic potential.108 Although there is little evidence of zoonotic transmission,84 owners, especially the immunosuppressed, should be advised to practice adequate hygiene when handling cats with suspect chlamydial conjunctivitis. Like FHV-1, C. felis prefers to replicate in epithelial cells; however, it appears to do so in a more diverse range of tissues than does FHV-1, including rectal and vaginal epithelia, as well as lung, spleen, liver, peritoneum, and kidneys.185 This knowledge helps explain why systemic therapy is now recommended for cats with chlamydial conjunctivitis, even if no extraocular signs are noted.
After an incubation period of approximately 3 to 5 days, cats develop conjunctivitis, mild fever, and sometimes submandibular lymphadenopathy. In contrast to FHV-1, respiratory signs are mild to absent, and the clinical disease course tends to be more insidious and persistent but not lifelong. The typical clinical presentation is periods of chronic mild conjunctivitis alternating with quiescent phases. Many cats shed chlamydial organisms for at least 60 days. Although the organisms may be spontaneously cleared, many cats require appropriate therapy for complete elimination. This helps explain why treatment of in-contact cats is often recommended for individual cats with chlamydial conjunctivitis. Co-infection with FHV-1 and C. felis appears to be very uncommon.24,109,155,195,196
Diagnosis
A major paradox exists with respect to the diagnosis of FHV-1.118 Cats experiencing primary FHV-1 infection shed virus in sufficient quantities that viral detection is relatively easy. However, clinical signs during this phase of infection tend to be characteristic and self-limiting, making definitive diagnosis less necessary. By contrast, the diversity and ambiguity of clinical signs in more chronic FHV-1–associated syndromes makes viral identification more desirable, but the elusive nature of the virus in these syndromes makes this difficult. Indeed, the diagnosis of FHV-1 in individual cats represents one of the greatest challenges in the management of chronic FHV-1–related diseases. The situation is equally challenging for C. felis. Culture is the gold-standard method of diagnosis; however, this requires special transport media and conditions and rigorous culture techniques and is expensive. The increased sensitivity and ease of organism detection using PCR have made it a preferred technique; however, it appears that many chlamydial organisms may not be detected by standard PCR assay.196 For these reasons clinical judgment using assessment of a number of helpful, but not pathognomonic, characteristics (Table 29-2) followed by response to appropriate therapeutic trials may be preferred.
TABLE 29-2 Comparison of the Biological Behavior of Chlamydophila felis and Feline Herpesvirus-1
| Feline herpesvirus-1 | Chlamydophila felis | |
|---|---|---|
| Organism | Obligate intracellular alphaherpesvirus | Obligate intracellular gram-negative bacterium |
| Strains | Few; biologically similar | Multiple; variable virulence |
| Environmental stability | 12-18 hours | A few days |
| Preferred replication site | Conjunctival epithelium | Conjunctival epithelium |
| Infection | Macrodroplets | Macrodroplets |
| Carrier state | Lifelong latency within trigeminal ganglia | Persistent (6-9 months) ocular and nonocular infections |
| Reinfection | Unlikely | Common |
| Subclinical shedding | More likely | Less likely |
Antiviral Treatment
Several antiviral drugs have been studied for their efficacy against FHV-1 and their safety in cats. However, it is critical to realize that because they were developed for use against human herpesviruses, they are not approved for use in cats, many are toxic to cats, some have dramatically reduced efficacy against FHV-1, and the pharmacokinetics and metabolism are often notably different in cats compared with humans. Provided that these limitations are understood and antiviral drugs are chosen in an evidence-based manner, response to therapy is an excellent way to support or refute the clinical diagnosis of FHV-1. Naturally, this must be tempered by an appreciation that both chlamydial and herpetic disease can wax and wane without therapy. The more commonly used and available antiviral drugs will be individually discussed in subsequent sections; however, some general comments regarding antiherpetic drugs are possible (Box 29-2).
Acyclovir has relatively low potency, poor bioavailability, and potential bone marrow toxicity when systemically administered to cats.142 In the authors’ opinion, the availability of safer and more effective drugs makes systemic administration of acyclovir difficult to justify. However, in countries where acyclovir is available as an ophthalmic ointment and can be applied very frequently, this may be effective and should circumvent systemic toxicity.203 In vitro data suggest that interferon exerts a synergistic effect with acyclovir that could permit an approximately eightfold reduction in acyclovir dose,198 but in vivo validation of these data is needed. Valacyclovir is a prodrug of acyclovir that is more efficiently absorbed from the feline gastrointestinal tract and is converted to acyclovir within the liver. Unfortunately, valacyclovir administration is associated with plasma acyclovir concentrations that are elevated to a point of causing fatal myelosuppression and renal and hepatic necrosis,135 and valacyclovir should never be used in cats. Penciclovir has a similar mechanism of action as acyclovir but with much more potent antiviral activity against FHV-1. Like acyclovir, its relatively poor bioavailability can probably be overcome by oral administration of the prodrug famciclovir. Pharmacokinetic, safety, and efficacy studies in cats reveal that famciclovir is remarkably effective and apparently safe in cats when administered at 90 mg/kg orally, thrice daily.121,187,188 Although clinical efficacy at lower doses has been reported,121 because of the nonlinear pharmacokinetics of famciclovir in the cat, further research is needed to establish the appropriate dose.
Cidofovir is a relatively new antiviral with good efficacy against FHV-1. When compounded with methylcarboxycellulose into a 0.5% ophthalmic solution and administered twice daily to experimentally infected cats, cidofovir was associated with reduced viral shedding and clinical disease.62 Although twice-daily dosing offers a clear advantage over all other topical antiherpetic drugs, cidofovir is not yet commercially available in Canada or the United States, and there are reports of its topical use in humans being associated with stenosis or cicatrization of the nasolacrimal drainage system components. As cidofovir becomes used more widely in cats, this potential side-effect should be monitored.
Lysine is an adjunctive therapy for FHV-1, but there are contradictory research data regarding its efficacy. In vitro data suggest that lysine exerts its antiviral effect by antagonism of arginine.116 In vivo studies demonstrated that administration of 500 mg lysine orally, twice daily, was associated with less severe conjunctivitis during primary infection179 and that 400 mg lysine orally, once daily, reduced viral shedding during latent infections.119 However, other clinical studies failed to show a positive effect or even demonstrated worsening of disease and increased viral shedding47,120,158 in two studies in which lysine was administered as a dietary supplement rather than in bolus form.47,120 For that reason cat owners should be advised to administer lysine as a twice-daily 500-mg bolus rather than by sprinkling it on food. Because lysine appears to be most effective when initiated before clinical disease, it may be most useful when administered indefinitely to cats experiencing repeated recrudescent disease,179 rather than being administered only during periods of active disease.
The interferons are a group of cytokines that have diverse immunologic and antiviral functions largely through limiting cell-to-cell viral spread. Although interferons may play important physiologic roles in the control of viral infections, data regarding potential therapeutic applications are conflicting. In vitro, interferon significantly reduced FHV-1 titer or cytopathic effect (or both) without detectable cytotoxic changes in the host cell lines162,170 and was associated with a nearly eightfold reduction in the required dose of acyclovir, especially when introduced to the viral culture before infection.198 However, there are relatively few peer-reviewed, placebo-controlled, prospective clinical trials of interferon administration in cats. Those that exist show minimal or no beneficial effects.76 Further research is necessary to determine dosage, timing, and efficacy (if any) of this group of compounds, especially in the more chronic or recrudescent infections.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree