CHAPTER 28 Oncology
Basic Approach to the Feline Cancer Patient
Signs of Cancer
All too commonly, the veterinarian sees a feline patient after clinical signs have exceeded the client’s perception of health. Subtle behavioral changes may occur long before overt clinical signs appear. These changes vary depending on the type of malignancy and which body systems are most affected. General changes in activity, appetite, and litter box use are perhaps the most common first indications that a disease process is under way (see Box 37-3).
Screening Diagnostics
General health screening is the first step for the ill cat or a cat whose clinical signs designate the location of the disease. A complete blood count (CBC) can reveal hematologic malignancies and bone marrow–infiltrating diseases. The CBC is also an excellent way to primitively assess the innate immune system. Biochemistry data aid in the evaluation of general organ function as well as screening for hepatic lipidosis, the most common secondary disease.1 General body cavity imaging is necessary because not all malignancies lead to biochemical alterations. Complete staging generally includes both abdominal and thoracic radiographs and potentially abdominal ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI).
Biopsy Techniques
Punch Biopsy and Needle Biopsy
Technique
When a biopsy punch is used, cutaneous and subcutaneous samples can be acquired transdermally or through a small skin incision. The biopsy punch is twisted, always in the same direction, into the lesion to the desired depth within the mass. With Metzenbaum scissors the sample is cut away from adherent underlying tissues. The sample is placed in formalin at a ratio of 1 : 10 (tumor : formalin) and submitted to a pathologist, who will provide a complete microscopic description, diagnosis, grade, margin description, and mitotic index as indicated. For a needle biopsy, a Tru-Cut biopsy needle is required. A Tru-Cut biopsy requires a special tool. This needle biopsy tool is inserted into the lesion. The core of the needle biopsy device is extended into the tissue mass. The sheath of the needle biopsy device is then advanced, and a portion of tissue is cut free within the notch of the core. Multiple specimens should be collected. Automatic firing devices can speed the collection of each sample. However, these automated, spring-loaded devices can be too vigorous for internal organs, causing organ damage in smaller patients.3
1 Armstrong PJ, Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract. 2009;39:599.
2 Hershey AE, Sorenmo KU, Hendrick MJ, et al. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986-1996). J Am Vet Med Assoc. 2000;216:58-61.
3 Proot SJ, Rothuizen J. High complication rate of an automatic Tru-Cut biopsy gun device for liver biopsy in cats. J Vet Intern Med. 2006;20:1327.
Chemotherapy for the Feline Cancer Patient
The aim of this section is to provide information regarding the safe handling and administration of chemotherapy as well as information regarding dosing and potential toxicoses of some of the chemotherapy agents commonly administered to cats. Additional resources are found in Box 28-1.
BOX 28-1 Additional Resources
ASHP guidelines on handling hazardous drugs, Am J Health Syst Pharm 63:1172-1191, 2006. http://www.ashp.org/DocLibrary/BestPractices/PrepGdlHazDrugs.aspx.
Burroughs GE, Connor TH, McDiarmid MA et al: Preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings, National Institute for Occupational Safety and Health, 2004. http://www.cdc.gov/niosh/docs/2004-165/.
Chemotherapy and managing oncologic emergencies. In Henry CJ, Higginbotham ML: Cancer management in small animal practice, St. Louis, 2010, Saunders Elsevier, pp 101-135.
Chun R, Garrett LD, Vail DM: Cancer chemotherapy. In Withrow SJ, Vail DM: Withrow and MacEwen’s small animal clinical oncology, ed 4, St. Louis, 2007, Saunders Elsevier, pp 163-192.
Thamm DH, Vail DM: Aftershocks of cancer chemotherapy: managing adverse effects, J Am Anim Hosp Assoc 43:1, 2007.
Chemotherapy Preparation
The risk of exposure of personnel to chemotherapy drugs is greatest during preparation and administration. These cytotoxic drugs may have mutagenic and carcinogenic effects. All staff members should be aware of the risks of exposure to these drugs and follow protocols to minimize these risks. Clinics that administer chemotherapy should have a written set of guidelines for the safe handling of chemotherapy drugs and plans for managing chemotherapy spills or other exposures.1,6 Areas where chemotherapy is prepared and administered should be clearly marked, and traffic through those areas should be limited. Storage or consumption of food and beverages, including gum chewing, should be prohibited in these areas to prevent accidental ingestion. Cytotoxic drugs should be stored separately from other medications and their location clearly identified.
Facilities where injectable chemotherapy agents are prepared ideally should have a class II biologic safety cabinet that is vented to the outside and located in a space designated for chemotherapy preparation. The area should be free of clutter, and workroom surfaces should be disinfected with bleach. The work surface should be covered with an absorbent pad with a nonporous backing to help contain any spills that do occur. Compounding pharmacies can be used to prepare drugs for administration if space and proper equipment are cost prohibitive for the clinic. Items required for preparation of antineoplastic drugs are listed in Box 28-2. Personal protective equipment (PPE), including gloves, gown, protective eyewear, shoe covers, and a respirator or heavy-duty mask, should be worn when preparing cytotoxic drugs. Gowns should be made of a low-permeability fabric and have a closed front and long sleeves with elastic cuffs. Chemotherapy gloves or two pairs of latex, nitrile, or neoprene gloves are recommended, and gloves should be worn over the cuffs of the gown. Powder-free gloves should be used because the powder may absorb contaminants and increase the risk of exposure. A chemotherapy spill kit should be easily accessible in all areas where chemotherapy is handled. Box 28-3 lists the items necessary for a chemotherapy spill kit; alternatively, ready-made spill kits can also be purchased.
BOX 28-2 Requirements for Chemotherapy Preparation and Administration
Chemotherapy Preparation
• Personal protective equipment
• Absorbent pad with nonporous backing to cover preparation surface
• Needles, Luer-Lok syringes, syringe caps
• Drug-containment devices such as PhaSeal or ONGUARD
• Appropriate diluent, as indicated on the drug package insert
• Sealable plastic bag for transporting chemotherapy
• Dedicated chemotherapy sharps container
Chemotherapy Administration
BOX 28-3 Chemotherapy Spill Kit Requirements
Reconstitution of chemotherapy drugs may cause aerosolization of the agent. For this reason devices designed to prevent aerosolization should be used. Chemotherapy dispensing pins are venting devices with a 0.22-micron filter that reduce pressure in the chemotherapy vial when reconstituting and dispensing chemotherapy drugs, thereby decreasing the risk of spraying or spillage. Optimally, a closed-system drug delivery device such as PhaSeal (Carmel Pharma, Columbus, Ohio) or a contained medication system such as ONGUARD (B Braun, Bethlehem, Penn.) can be used. Closed-system devices prevent aerosolization of drugs and provide leak-free (dry) connection between the vial, syringe, infusion set, and the cat; they have been demonstrated to decrease surface contamination and personnel exposure.43,44 Regardless of the venting or delivery device used, the use of Luer-Lok syringes is essential when reconstituting or drawing up chemotherapy drugs to prevent accidental disconnection of the syringe from the delivery system. Intravenous fluid lines should be primed before the addition of the chemotherapy agent to the infusion bag to prevent contamination when priming the line. Once chemotherapy preparation is complete, the drug should be placed in a sealable plastic bag for transport to the administration area to contain any leaks or spills that may occur during transportation. Suppliers of chemotherapy equipment are listed in Box 28-4.
Chemotherapy Dosing and Administration
Personnel should be instructed in the proper handling and disposal of waste from chemotherapy patients. Owners should be given written instructions regarding these as well. Depending on the drug administered, urine, feces, saliva, and vomitus may contain small amounts of chemotherapy agents and their metabolites for as long as 72 hours after administration; chemotherapy drugs that undergo substantial protein binding may not be fully excreted for as long as 21 days after treatment.26 While the cat is in the hospital, cage cards identifying chemotherapy patients should be used to alert staff members responsible for cleaning soiled cages. Soiled linens should be washed separately, and disposable gloves should be worn when cleaning up urine, feces, or vomitus during this time. Litter boxes should be changed daily for several days after treatment, and use of litter-box liners may help prevent aerosolizing chemotherapy metabolites. Cats in multicat households do not need to be separated because there is no reported risk associated with sharing litter boxes or food dishes.
Intravenous Administration
Box 28-2 lists the equipment necessary for intravenous administration of cytotoxic drugs. PPE, as previously described, should be worn by the individual administering the drug and any personnel involved in restraint of the cat. An absorbent pad with a plastic backing should be placed underneath the limb into which the drug is to be delivered. A butterfly catheter can be used for cooperative cats receiving small chemotherapy volumes (<3 mL). For larger volumes an indwelling catheter should be placed and the cat closely monitored throughout the infusion. The catheter should be flushed thoroughly with nonheparinized saline before administration to ensure its patency. Chemotherapy agents should not be infused with an intravenous fluid pump. Chemotherapy drugs should be administered by slow gravity drip or manual syringe infusion over the recommended time of administration. The fluid bag should be lowered beneath the cat every few minutes to ensure that blood is able to flow back in the catheter. If the drug is administered through a syringe, the plunger should be aspirated back several times during administration of the drug to ensure that blood still appears in the hub of the catheter. The catheter site should be monitored throughout administration and administration discontinued if any swelling at the catheter site is observed. Once the chemotherapy infusion is complete, the catheter should be flushed again with nonheparinized saline before removal. A light bandage should be placed over the catheter site after removal.
Intracavitary Administration
Chemotherapy agents such as carboplatin and mitoxantrone may be administered in the pleural or peritoneal space to mitigate malignant effusions. The intracavitary dose of chemotherapy is generally the same as that administered intravenously and can be administered into a single body cavity or divided between the thorax and the abdomen. The chemotherapy drug should be diluted with the appropriate diluent to a maximal volume of 60 mL for intrathoracic administration and 250 mL for intraabdominal administration.34 As appropriate, effusions should be drained from the cavity before instillation of the chemotherapy agent.
General Adverse Effects of Chemotherapy
Gastrointestinal
Chemotherapy agents can be directly cytotoxic to the intestinal epithelial crypt cells resulting in gastrointestinal side effects about 2 to 5 days after administration. Less commonly, some chemotherapy agents may cause release of 5-HT from enterochromaffin cells in the gastrointestinal tract, which binds to 5-HT3 receptors on peripherally vagal nerves or centrally in the chemoreceptor trigger zone. 5-HT3–mediated nausea and vomiting tend to occur within 24 hours of chemotherapy administration. These side effects may include mild to severe inappetence, nausea, vomiting, and diarrhea. For the majority of cats, gastrointestinal side effects secondary to chemotherapy are mild and self-limiting and often can be easily managed by owners at home with the administration of oral antinausea and antidiarrheal drugs on an as-needed basis. Oral antiemetics such as metoclopramide, ondansetron, and maropitant (see Table 28-5) can be dispensed at the time of the first chemotherapy appointment, whereupon owners should be instructed when to give the medications. Medications such as tylosin and metronidazole can be dispensed in a similar manner for owners to administer in the case of soft stools or diarrhea. Probiotics can also be administered throughout the duration of chemotherapy and have been anecdotally reported to decrease the frequency and severity of chemotherapy-induced diarrhea.
Extravasation
Signs of extravasation may include pain, erythema, moist dermatitis, and necrosis and may appear 1 to 10 days after extravasation of the drug.28 If extravasation is suspected at the time of administration, the infusion should be stopped immediately. An attempt should be made to aspirate the drug with up to 5 mL of blood back into the syringe. The catheter is removed once this has been accomplished. Recommendations regarding additional treatment are generally extrapolated from experiences in human oncology and are based on the type of drug that was extravasated. In the case of vinca alkaloid extravasation, warm, dry compresses can be applied for several hours and hyaluronidase injected into the local site.10 The volume of hyaluronidase injected should equal the volume of drug extravasated. Administration of dexrazoxane (Zinecard), a free-radical scavenger marketed to prevent doxorubicin-associated cardiotoxicity in humans, is indicated in the case of doxorubicin extravasation. The recommended dose is 1 : 10 of vesicant to dexrazoxane, and this should be administered intravenously through a separate catheter within 6 hours of extravasation.10,30 Dexrazoxane is expensive and may be too costly for practitioners to stock. Availability at a local human hospital should be investigated because timely administration (within 3 to 6 hours) after extravasation may help mitigate tissue necrosis. Alternatively, topically applied dimethyl sulfoxide (DMSO) to the site of extravasation may help minimize tissue damage as well.51 Aggressive surgical débridement may be required to manage severe cases of perivascular necrosis.
Commonly Used Chemotherapy Drugs
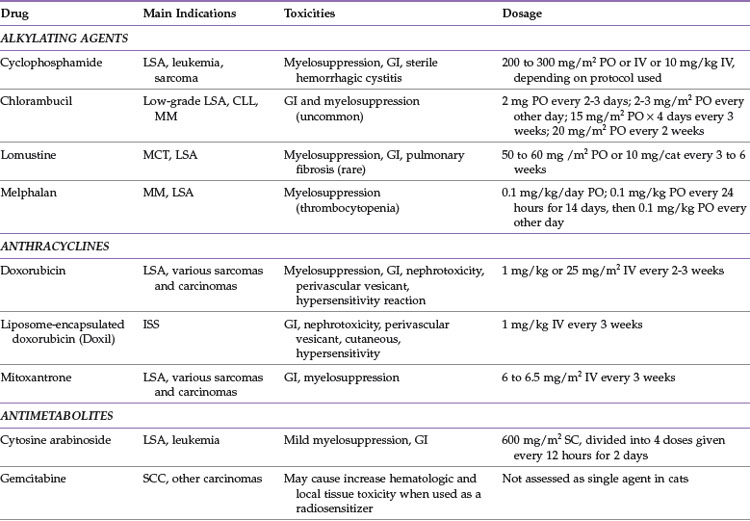
This section deals with chemotherapy agents commonly used for treating cats with cancer, as well as some newer agents about which limited information is known. Table 28-1 summarizes these chemotherapy drugs, common indications, dosages, and associated toxicities.
Alkylating Agents
Chlorambucil (Leukeran)
Chlorambucil is an orally administered DNA alkylating agent that is used to treat low-grade lymphoma, chronic lymphocytic leukemia, and, less commonly, multiple myeloma. Reported dosages include 2 mg orally every 2 or 3 days, 2 to 4 mg/m2 orally every other day, 15 mg/m2 orally every day for 4 consecutive days once every 3 weeks, and 20 mg/m2 orally once every 2 weeks.13,23,49
Chlorambucil is generally well tolerated, and gastrointestinal signs are uncommon. Myelosuppression may occur after prolonged used. Rare toxicities may include neurotoxicity, which has been reported in a single cat, and there may be an increased risk of developing a second malignancy with prolonged therapy.4,49
Cyclophosphamide (Cytoxan)
Common side effects include myelosuppression and gastrointestinal toxicity. Less commonly, sterile hemorrhagic cystitis may develop secondary to cyclophosphamide administration.8 If signs of hematuria, pollakiuria, or stranguria are observed in a cat that has been recently treated with cyclophosphamide, a urinalysis and urine culture should be performed. If the urine culture is negative, a presumptive diagnosis of sterile hemorrhagic cystitis can be made, and cyclophosphamide therapy should be discontinued permanently.
Lomustine (CCNU, CeeNU)
Lomustine is an oral DNA alkylating agent that is most frequently used against mast cell tumors and lymphoma.11,41,42 Because of its ability to cross the blood–brain barrier, it is also used to treat brain tumors, but efficacy against these tumor types is not documented in cats. It may be efficacious against fibrosarcoma and multiple myeloma.11 Lomustine is administered at 50 to 60 mg/m2 or 10 mg/cat orally once every 3 to 6 weeks.11,41,42 It may be necessary to compound capsules to smaller sizes for more accurate dosing because the 10 mg/cat dose may underdose or overdose some cats.
Myelosuppression, particularly neutropenia, is the dose-limiting toxicity for lomustine. Severe and persistent thrombocytopenia can occur that warrants discontinuation of the drug if platelet numbers do not return to normal levels in 6 weeks. Gastrointestinal signs can occur with this drug as well. Hepatotoxicity has not been reported in cats to date, but routine monitoring of liver enzymes is still recommended. Pulmonary fibrosis can occur in people treated with CCNU, and a report of pulmonary fibrosis developing after chronic CCNU therapy exists for a single cat.46 Renal toxicity is an uncommon side effect in humans and has not been reported in cats, but routine monitoring of kidney values should be performed for cats with documented renal insufficiency that are receiving CCNU.
Melphalan
Melphalan is an oral DNA alkylating agent that is most commonly used in treatment of multiple myeloma and occasionally lymphoma. Melphalan can be administered at 0.1 mg/kg orally once daily or 0.1 mg/kg orally daily for 14 days followed by 0.1 mg/kg orally every other day.5,7 Tablets may need to be compounded for more accurate dosing because they should not be split.
Anthracyclines
Doxorubicin (Adriamycin)
More common side effects of doxorubicin administration include gastrointestinal signs and myelosuppression. Doxorubicin is a potent vesicant, and utmost caution should be used to ensure that extravasation does not occur during administration. Cumulative nephrotoxicity may occur with doxorubicin administration, and this drug should not be used in cats with renal insufficiency.36 Renal values and urine specific gravity should be monitored routinely in cats receiving doxorubicin and the drug discontinued if isosthenuria or azotemia occur. Cats can also have an acute hypersensitivity reaction with doxorubicin, and some practitioners routinely premedicate cats receiving doxorubicin with diphenhydramine or dexamethasone SP (or both). Regardless of whether premedication with antihistamines or corticosteroids is performed, the cat should be closely monitored during the infusion for any indications of a hypersensitivity reaction, such as head shaking, erythema of the pinna or mucous membranes, facial swelling, dyspnea, and agitation. Cumulative cardiotoxicity with doxorubicin administration is well documented in humans and dogs but is infrequent in cats.36 Administration of doxorubicin to cats with underlying cardiac disease is discouraged, and some practitioners recommend not exceeding cumulative doses of 180 to 240 mg/m2 in cats with normal cardiac function.
Liposmal-Encapsulated Doxorubicin (Doxil)
Liposomal-encapsulated doxorubicin was formulated to avoid the significant cardiotoxicity in humans that limits doxorubicin administration. This drug may have efficacy against similar tumor types as doxorubicin, including injection-site sarcomas, and is dosed at 1 mg/kg intravenously every 3 weeks.40
Liposomal-encapsulated doxorubicin has a similar toxicity profile in cats as native doxorubicin. Gastrointestinal side effects are generally mild and self-limiting. Liposomal-encapsulated doxorubicin is associated with nephrotoxicity, and renal function should be closely monitored after administration of the drug.40 It is a vesicant and should be administered only through a cleanly placed intravenous catheter. Cats may also develop a nonpainful alopecia and erythema with hyperpigmentation around their mouths and distal limbs.40 Cats may experience a hypersensitivity reaction characterized by salivation and bradycardia during their first treatment with liposomal-encapsulated doxorubicin; this can be managed with administration of diphenhydramine and dexamethasone SP.40 Intracavitary administration of liposomal-encapsulated doxorubicin has been reported in dogs but has not been evaluated in cats.
Mitoxantrone
Mitoxantrone is an anthracycline derivative that exerts its cytotoxic effects by inhibiting topoisomerase II. This drug likely has a similar antitumor profile to doxorubicin and may be efficacious against lymphoma and various carcinomas and sarcomas.35 The mitoxantrone dosage is 6 to 6.5 mg/m2 intravenously every 3 weeks. The drug is diluted in 20 to 50 mL 0.9% NaCl before intravenous administration over 10 to 15 minutes. Mitoxantrone can be administered in the pleural or peritoneal space to help alleviate malignant effusions.
The most common adverse effects observed with mitoxantrone are mild, self-limiting gastrointestinal signs and myelosuppression. Unlike doxorubicin, mitoxantrone does not induce hypersensitivity reactions and is not as potent a vesicant if extravasated. Owners should be cautioned that urine and sclera may be blue-tinged after administration.7
Antimetabolites
Cytosine Arabinoside (Cytarabine, Cytosar-U, ara-C)
Cytosine arabinoside is a deoxycytidine analog that interferes with DNA synthesis through DNA polymerase inhibition. Because this drug is cell cycle specific with an extremely short plasma half-life, it is most efficacious when administered by constant-rate infusion or with the dose divided into twice-daily subcutaneous injections over several days. Cytosine arabinoside is used in cats primarily to treat lymphoma or leukemia, particularly when there is central nervous system (CNS) involvement, because of the ability of the drug to cross the blood–brain barrier. Cytosine arabinoside is often substituted for cyclophosphamide for treatment of renal lymphoma because CNS involvement occurs in approximately 40% of these cases.33 The dosage of cytosine arabinoside is 600 mg/m2 divided into four doses administered subcutaneously twice daily for 2 days or administered as a constant-rate infusion at 300 mg/m2 per day for 2 days.7
Gemcitabine
Gemcitabine is an analog of deoxycytidine that is activated intracellularly, resulting in DNA synthesis inhibition. There is limited information on the use of gemcitabine as a single agent in cats. It has been used as a radiation sensitizer in the treatment of oral squamous cell carcinoma (SCC) and also in combination with carboplatin as therapy for various carcinomas.22,29,31 This drug is expensive compared with other chemotherapy drugs, and optimal dosing in cats is not known at this time.
Adverse effects when administered intravenously at 2 mg/kg weekly in conjunction with carboplatin included moderate gastrointestinal toxicity and myelosuppression.31 When administered intravenously at 25 mg/m2 twice weekly as a radiation sensitizer, significant hematologic and local tissue toxicity occurred.29 Additional research investigating efficacy, dosage, and toxicity of gemcitabine as a single agent drug is necessary before it can be used routinely in cats with cancer.
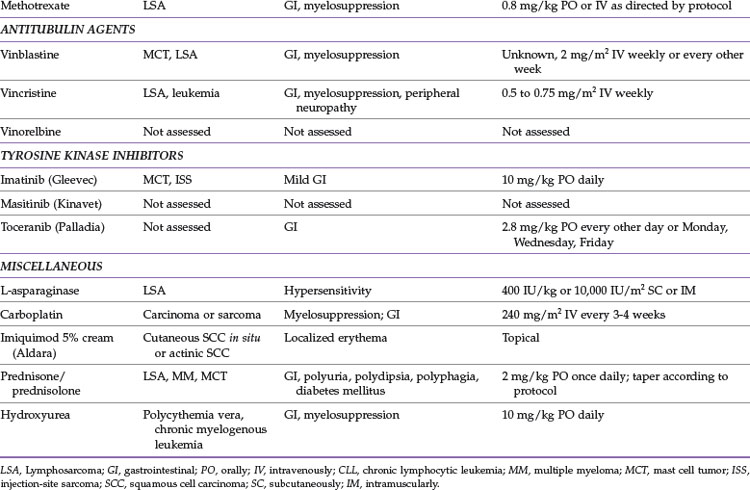
Methotrexate
Methotrexate is an antifolate that inhibits dihydrofolate reductase, thereby blocking DNA synthesis. It has been used primarily in combination chemotherapy protocols for lymphoma at a dosage of 0.8 mg/kg weekly, either intravenously or orally.32,33,45
Antitubulin Agents
Vincristine
Vincristine is used commonly to treat lymphoma and leukemia; reported dosages range from 0.5 to 0.75 mg/m2 given as an intravenous bolus once weekly. Peripheral neuropathy, which may present as constipation in cats, is uncommon but may occur with prolonged administration.7
Platinum Drugs
Carboplatin
Carboplatin is a platinum-derived alkylating agent that may have efficacy against various carcinomas and sarcomas.7,24 The most common route of administration is intravenous, but carboplatin can also be administered intracavitarily, in the thorax or abdomen, and intralesionally. Carboplatin is administered intravenously at 240 mg/m2 every 3 to 4 weeks. Dosing of carboplatin on the basis of glomerular filtration rate has been investigated and may allow for more appropriate dosing in cats, but this is clinically impractical for most practitioners.2,3 Carboplatin can also be used intralesionally for facial SCC. General anesthesia is required for treatment, and carboplatin is administered at 1.5 mg/cm3 in purified sesame oil emulsion injected every 0.5 cm in the tumor and adjacent tissues weekly for up to four treatments.53 Carboplatin has been reported to be administered intracavitarily at 180 to 200 mg/m2 to help alleviate malignant effusions; however, information regarding efficacy is limited.47,48
Myelosuppression is the dose-limiting toxicity of carboplatin. Neutrophil and platelet nadirs generally occur 2 to 3 weeks after administration.24 Gastrointestinal signs occur less commonly with carboplatin. Because carboplatin is excreted by the kidneys, it is important to assess renal function (blood urea nitrogen, creatinine, and urine specific gravity) before each treatment. Carboplatin is not commonly directly nephrotoxic, but decreased excretion occurs with decreased renal function, thereby increasing the likelihood of toxicity.
Tyrosine Kinase Inhibitors
Imatinib (Gleevec)
Imatinib has been used to treat feline cutaneous, splenic, and disseminated mast cell tumors at doses at 10 to 15 mg/kg orally once daily. The most common side effects noted are mild gastrointestinal upset.20,21,27
Toceranib (Palladia)
Information regarding use of toceranib in cats is limited. Preliminary results suggest that dosage of 2.8 mg/kg orally every other day or on a Monday/Wednesday/Friday schedule may have some efficacy against oral SCC and injection-site sarcomas.19 Most common side effects are gastrointestinal, particularly anorexia with weight loss and vomiting.19
Masitinib (Kinavet CA-1)
Masitinib has been administered to healthy cats at a dosage of 50 mg/cat orally every 24 to 48 hours and was well tolerated over a 4-week period.9 Gastrointestinal toxicosis was most common, with neutropenia and proteinuria occurring less frequently.9 There is no information at this time regarding dosage, safety, and efficacy for masitinib in cats with cancer.
Miscellaneous
Hydroxyurea
Hydroxyurea is an oral chemotherapy agent that suppresses proliferation of myeloid, erythroid, and platelet precursors by inhibiting DNA synthesis.37 The main indications for hydroxyurea are treatment of polycythemia vera and chronic myelogenous leukemia. The recommended dosage is 10 mg/kg orally once daily.7
Side effects associated with hydroxyurea therapy in cats may include myelosuppression and gastrointestinal toxicity.7
Imiquimod (Aldara)
Imiquimod 5% cream (Aldara) is a topical immune response modifier that has been shown to have antitumor effects by enhancement of both innate and cell-mediated immunity. Data are limited at this time, but imiquimod 5% cream may be effective in treating multifocal, cutaneous SCC in situ or actinic (solar-induced) SCC in cats.14,38 Reported topical application schedules range from once daily to three times a week on affected areas.
Adverse events that have been reported include mild erythema at the site of application.14,38 Potential systemic toxicities have been reported as well, and these are likely secondary to ingestion of imiquimod 5% cream by the cat because the drug should not have systemic effects when applied topically. These side effects include mild gastrointestinal upset, neutropenia, and elevated liver enzymes.14 It is recommended that routine monitoring of CBC and biochemistry panel be performed every 4 to 8 weeks in cats treated with imiquimod 5% until more information is known about this drug in cats.
Prednisone and Prednisolone
Prednisolone and its prodrug prednisone are glucocorticoids frequently used in veterinary oncology. In most species prednisone is converted to prednisolone in the liver, but there is some concern that this step does not occur efficiently in some cats. Therefore use of prednisolone rather than prednisone is recommended in this species.39 Prednisolone is commonly used in multidrug chemotherapy protocols, and there is evidence that prednisolone has activity against lymphoma, plasma cell tumors, and mast cell tumors.* The antitumor dose of prednisolone is 2 mg/kg orally once daily. This dose is tapered over approximately 1 month and then generally discontinued when used in combination chemotherapy protocols. Other common uses for prednisolone include decreasing edema associated with tumors of the CNS and as an antiinflammatory for pain control in cats that cannot tolerate administration of nonsteroidal antiinflammatory drugs. For these indications, prednisolone is generally administered at antiinflammatory dosages (0.5 to 1 mg/kg orally once daily).
Prednisolone is generally well tolerated in cats. Adverse effects may include polyphagia, polydipsia, polyuria, and gastrointestinal irritation. Rarely, chronic high-dose prednisolone therapy may lead to development of diabetes mellitus in cats.12
1 ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm. 2006;63:1172.
2 Bailey DB, Rassnick KM, Dykes NL, et al. Phase I evaluation of carboplatin by use of a dosing strategy based on a targeted area under the platinum concentration-versus-time curve and individual glomerular filtration rate in cats with tumors. Am J Vet Res. 2009;70:770.
3 Bailey DB, Rassnick KM, Erb HN, et al. Effect of glomerular filtration rate on clearance and myelotoxicity of carboplatin in cats with tumors. Am J Vet Res. 2004;65:1502.
4 Benitah N, de Lorimier L-P, Gaspar M, et al. Chlorambucil-induced myoclonus in a cat with lymphoma. J Am Anim Hosp Assoc. 2003;39:283.
5 Betts GJ, Clarke SL, Richards HE, et al. Regulating the immune response to tumours. Adv Drug Del Rev. 2006;58:948.
6 Burroughs GE, Connor TH, McDiarmid MA, et al. Preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings in Cincinnati, OH. National Institute for Occupational Safety and Health. 2004.
7 Chun R, Garrett LD, Vail DM. Cancer chemotherapy. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen’s small animal clinical oncology. ed 4. St Louis: Saunders Elsevier; 2007:163-192.
8 Crow SE, Theilen GH, Madewell BR, et al. Cyclophosphamide-induced cystitis in the dog and cat. J Am Vet Med Assoc. 1977;171:259.
9 Daly M, Sheppard S, Cohen N, et al. Safety of masitinib mesylate in healthy cats. J Vet Intern Med. 2011;25:297.
10 Dhaliwal RS. Managing oncologic emergencies. In: Henry CJ, Higginbotham ML, editors. Cancer managment in small animal practice. St Louis, Mo: Saunders Elsevier; 2010:122-135.
11 Fan TM, Kitchell BE, Dhaliwal RS, et al. Hematological toxicity and therapeutic efficacy of lomustine in 20 tumor-bearing cats: critical assessment of a practical dosing regimen. J Am Anim Hosp Assoc. 2002;38:357.
12 Feldman EC, Nelson RW. Feline diabetes mellitus. In: Feldman EC, Nelson RW, editors. Canine and feline endocrinology and reproduction. ed 3. St Louis: Saunders; 2004:539-579.
13 Fondacaro J, Ritcher K, Carpenter J, et al. Feline gastrointestinal lymphoma: 67 cases (1988-1996). Eur J Comp Gastroenterol. 1999;4:5.
14 Gill VL, Bergman PJ, Baer KE, et al. Use of imiquimod 5% cream (AldaraTM) in cats with multicentric squamous cell carcinoma in situ: 12 cases (2002-2005). Vet Comp Oncol. 2008;6:55.
15 Hadden AG, Cotter SM, Rand W, et al. Efficacy and toxicosis of velcap-c treatment of lymphoma in cats. J Vet Intern Med. 2008;22:153.
16 Hanna F. Multiple myelomas in cats. J Feline Med Surg. 2005;7:275.
17 Harvey HJ, MacEwen EG, Hayes AA. Neurotoxicosis associated with use of 5-fluorouracil in five dogs and one cat. J Am Vet Med Assoc. 1977;171:277.
18 Henness AM, Theilen GH, Madewell BR, et al. Neurotoxicosis associated with use of 5-florouracil. J Am Vet Med Assoc. 1977;171:692.
19 Hohenhaus AE. Biological activity and adverse event profile in cats treated with toceranib phosphate. San Diego, Calif: Annual Conference of the Veterinary Cancer Society; 2010. p 64
20 Isotani M, Tamura K, Yagihara H, et al. Identification of a c-kit exon 8 internal tandem duplication in a feline mast cell tumor case and its favorable response to the tyrosine kinase inhibitor imatinib mesylate. Vet Immunol Immunopathol. 2006;114:168.
21 Isotani M, Yamada O, Lachowicz JL, et al. Mutations in the fifth immunoglobulin-like domain of kit are common and potentially sensitive to imatinib mesylate in feline mast cell tumours. Br J Haematol. 2010;148:144.
22 Jones PD, de Lorimier L-P, Kitchell BE, et al. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc. 2003;39:463.
23 Kiselow MA, Rassnick KM, McDonough SP, et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995-2005). J Am Vet Med Assoc. 2008;232:405.
24 Kisseberth WC, Vail DM, Yaissle J, et al. Phase I clinical evaluation of carboplatin in tumor-bearing cats: a veterinary cooperative oncology group study. J Vet Intern Med. 2008;22:83.
25 Knapp DW, Richardson RC, DeNicola DB, et al. Cisplatin toxicity in cats. J Vet Intern Med. 1987;1:29.
26 Knobloch A, Mohring SAI, Eberle N, et al. Drug residues in serum of dogs receiving anticancer chemotherapy. J Vet Intern Med. 2010;24:379.
27 Lachowicz JL, Post GS, Brodsky E. A phase I clinical trial evaluating imatinib mesylate (Gleevec) in tumor-bearing cats. J Vet Intern Med. 2005;19:860.
28 Lana SE, Dobson JM. Principles of chemotherapy. In: Dobson JM, Lascelles BDX, editors. BSAVA manual of canine and feline oncology. ed 3. Gloucester, UK: British Small Animal Veterinary Association; 2011:60-79.
29 LeBlanc AK, LaDue TA, Turrel JM, et al. Unexpected toxicity following use of gemcitabine as a radiosensitizer in head and neck carcinomas: a veterinary radiation therapy oncology group pilot study. Vet Radiol Ultrasound. 2004;45:466.
30 Mahoney JA, Bergman PJ, Camps-Palau MA, et al. Treatment of doxorubicin extravasation with intravenous dexrazoxane in a cat. J Vet Intern Med. 2007;21:872.
31 Martinez-Ruzafa I, Dominguez PA, Dervisis NG, et al. Tolerability of gemcitabine and carboplatin doublet therapy in cats with carcinomas. J Vet Intern Med. 2009;23:570.
32 Mooney S, Hayes AA, MacEwen EG, et al. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977-1981). J Am Vet Med Assoc. 1989;194:696.
33 Mooney S, Hayes AA, Matus RE, et al. Renal lymphoma in cats: 28 cases (1977-1884). J Am Vet Med Assoc. 1987;191:1473.
34 Ogilvie GK, Moore AS. Drug handling and adminsitration. In: Ogilvie GK, Moore AS, editors. Feline oncology: a comprehensive guide to compassionate care. Trenton, NJ: Veterinary Learning Systems; 2001:53-61.
35 Ogilvie GK, Moore AS, Obradovich JE, et al. Toxicoses and efficacy associated with administration of mitoxantrone to cats with malignant tumors. J Am Vet Med Assoc. 1993;202:1839.
36 O’Keefe DA, Sisson DD, Gelberg HB, et al. Systemic toxicity associated with doxorubicin administration in cats. J Vet Intern Med. 1993;7:309.
37 Paz-Ares L, Donehower RC, Chabner BA. Hydoxyurea. In: Chabner BA, Longo DL, editors. Cancer chemotherapy and biotherapy. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2006:229-236.
38 Peters-Kennedy J, Scott DW, Miller JrWH. Apparent clinical resolution of pinnal actinic keratoses and squamous cell carcinoma in a cat using topical imiquimod 5% cream. J Feline Med Surg. 2008;10:593.
39 Plumb DC. Plumb’s veterinary drug handbook, ed 6. Stockholm, Wisconsin: PharmaVet; 2008.
40 Poirier VJ, Thamm DH, Kurzman ID, et al. Liposome-encapsulated doxorubicin (Doxil) and doxorubicin in the treatment of vaccine-associated sarcoma in cats. J Vet Intern Med. 2002;16:726.
41 Rassnick KM, Gieger TL, Williams LE, et al. Phase I evaluation of CCNU (Lomustine) in tumor-bearing cats. J Vet Intern Med. 2001;15:196.
42 Rassnick KM, Williams LE, Kristal O, et al. Lomustine for treatment of mast cell tumors in cats: 38 cases (1999-2005). J Am Vet Med Assoc. 2008;232:1200.
43 Sessink PJ, Connor TH, Jorgenson JA, et al. Reduction in surface contamination with antineoplastic drugs in 22 hospital pharmacies in the US following implementation of a closed-system drug transfer device. J Oncol Pharm Pract. 2011;17:39.
44 Siderov J, Kirsa S, McLauchlan R. Reducing workplace cytotoxic surface contamination using a closed-system drug transfer device. J Oncol Pharm Pract. 2010;16:19.
45 Simon D, Eberle N, Laacke-Singer L, et al. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med. 2008;22:394.
46 Skorupski KA, Durham AC, Duda L, et al. Pulmonary fibrosis after high cumulative dose nitrosourea chemotherapy in a cat. Vet Comp Oncol. 2008;6:120.
47 Sparkes A, Murphy S, McConnell F, et al. Palliative intracavitary carboplatin therapy in a cat with suspected pleural mesothelioma. J Feline Med Surg. 2005;7:313.
48 Spugnini EP, Crispi S, Scarabello A, et al. Piroxicam and intracavitary platinum-based chemotherapy for the treatment of advanced mesothelioma in pets: preliminary observations. J Exp Clin Cancer Res. 2008;27:6.
49 Stein TJ, Pellin M, Steinberg H, et al. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc. 2010;46:413.
50 Teske E, van Straten G, van Noort R, et al. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med. 2002;16:179.
51 Thamm DH, Vail DM. Aftershocks of cancer chemotherapy: managing adverse effects. J Am Anim Hosp Assoc. 2007;43:1.
52 Theilen G. Adverse effect from use of 5% fluorouracil. J Am Vet Med Assoc. 1987;191:276.
53 Theon AP, VanVechten MK, Madewell BR. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am J Vet Res. 1996;57:205.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree