Chapter 31 Occupational Exposure to Zoonotic Simian Retroviruses: Health and Safety Implications for Persons Working with Nonhuman Primates
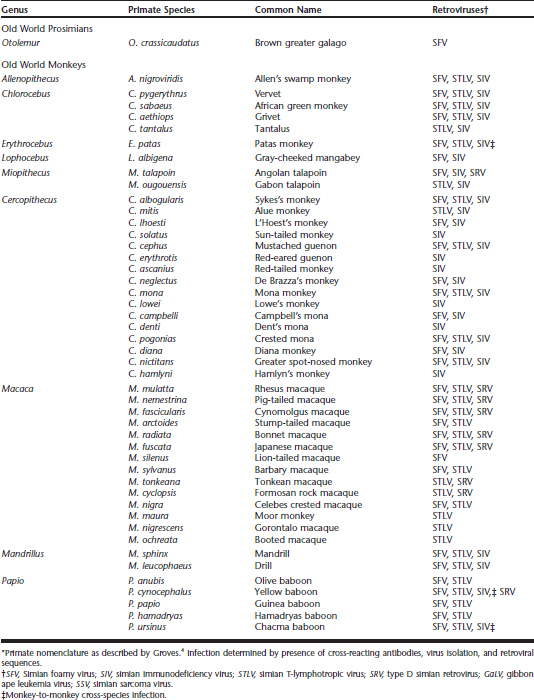
Nonhuman primates (NHPs) may be naturally infected with a plethora of viruses with zoonotic potential, including retroviruses Table 31-1. In recent years, concern for the prevalence and zoonotic risk potential of retroviruses in captive NHP collections at zoos has grown. In addition, concern has increased regarding the potential impact of these viruses on captive NHP populations, animal breeding, and transfer of specimens to new zoo collections.
SIMIAN RETROVIRUSES
All retrovirus genomes are composed of three major genes flanked by long terminal repeats (LTRs). The three major genes include the Gag or group specific antigen, which codes for the viral structural proteins; the polymerase (Pol) gene, which codes for the RT and integrase enzymes; and the envelope (Env) gene, which contains information for the transmembrane and surface proteins of the viral envelope. A smaller genomic region, Pro, is also present in all retroviruses and codes for the protease enzyme used in post-translational processing of viral proteins. Complex retroviruses also contain additional genes coding for regulatory proteins that control viral replication.
Simian retroviruses have received renewed public health interest since it was discovered that HIV types 1 and 2 (HIV-1 and HIV-2) originated zoonotically from cross-species transmission of SIV from infected chimpanzees (Pan troglodytes) and sooty mangabeys (Cercocebus atys) in central and western Africa, respectively.1,8,15 Similarly, HTLV type 1 (HTLV-1) has been shown to have originated from cross-species infection with STLV-1 from many primate species, and SFV and SRV infections have been recently observed in persons occupationally exposed to nonhuman primates.13,20 Together, these results have heightened awareness regarding the public health significance of these cross-species infections and raised animal and occupational health concerns over the retrovirus status of captive and wild NHPs.
SIMIAN IMMUNODEFICIENCY VIRUS (SIV)
Epizootiology
Simian immunodeficiency virus has been found in more than 30 species of both wild and captive primates.1,3,8,15 Seroprevalences as high as 76% have been seen in some naturally infected primates, with higher prevalence found in adults.1 Strains of SIV from different NHP species may be highly genetically diverse, with reports of 10 distinct phylogenetic lineages that share about 60% genetic identity.1,3,8,15 Most SIVs may grow in human peripheral blood mononuclear cells (PBMCs) in vitro, thus providing concern for the zoonotic potential of this virus.1
Natural transmission of SIV is thought to occur predominantly horizontally through sexual contact or bite wounds and less frequently by vertical transmission. Cross-species transmission of SIV to primates both in the wild and in captivity has been reported.1,3 Although New World primates and prosimians are not natural hosts to SIV, and in vitro studies demonstrate resistance of New World monkey cells to SIV infection, the in vivo susceptibility of New World monkeys and prosimians to SIV is unknown.17
Expression of Clinical Disease
Simian immunodeficiency virus usually produces lifelong and clinically inapparent infections in the naturally infected host species. However, when SIV infection jumps from its “natural” host species to a naive species, as was the case of viral transmission between African NHPs (sooty mangabeys) to Asian macaques (genus Macaca) in primate vivaria in the 1960s, immunosuppression and disease were demonstrated.10 Clinical signs of immunosuppression and disease from naturally occurring SIV are rare in African species but have been recognized in some primates after long-term infections.1 Asian primates, especially macaques, are not natural hosts of SIV but are very susceptible to potentially devastating acquired immunodeficiency syndrome (AIDS)–like disease when SIV is accidentally or experimentally introduced to a population.1,8
Clinical signs in susceptible populations range from acute epizootic infections to chronically, latently infected animals that may act as disease reservoirs and not show clinical signs for years. Lesions may include nonsuppurative histiocytic meningoencephalitis with syncytial giant cell formation, giant cell interstitial pneumonia, and disseminated giant cell disease. Persistent SIV infection may also result in lymphoproliferative diseases. Similar to HIV infection of humans, SIV infection of macaques has also been reported to cause severe lymphocyte depletion resulting in immunosuppression and the acquisition of a spectrum of opportunistic infections, such as cytomegalovirus, Candida, and Cryptosporidium, as well as the onset of clinical pathology associated with these agents.10
Interpretation of Diagnostic Assays
Because seroconversion may not be immediate after exposure and infection, new animals or animals with indeterminate serologic results may require testing on arrival and again in 3 to 6 months. During this quarantine period, polymerase chain reaction (PCR) testing for viral sequences with or without virus isolation using PBMCs or other tissues containing lymphocytes may also be needed to certify that an animal is SIV negative. Specific PCR primers for the suspected SIV strain may be used for diagnosis and to confirm the serologic results. Generic PCR primers from conserved regions in different SIV genomes may be necessary to confirm infection in cases in which a divergent virus or cross-species infection is suspected. Screening of free ranging NHPs for SIV may be done noninvasively using feces or urine specimens.1,3
Detection of genetically modified SIV/HIV (SHIV) recombinants, typically used as viral inocula in research studies using primates as models of HIV infection, may be complicated by the specific HIV or SIV genes contained in the genetic hybrid. For example, testing for SIV in this case should be restricted to primers located in the SIV portion of the SHIV hybrid. In addition, some SHIVs destroy CD4+ T cells so rapidly that an antibody response is not initiated, resulting in false-negative serologic results.13 Thus, molecular testing is needed to confirm infection in some SHIV-infected animals and in persons potentially exposed to these viruses.
Human Infection with SIV
As described earlier, cross-species transmission of SIVs from chimpanzees (SIVcpz) and sooty mangabeys (SIVsm) have been linked to the origin of the HIV-1 and HIV-2 epidemics, respectively.1,8 Approximately 40 million people worldwide are infected with HIV-1 and HIV-2, with over half in sub-Saharan Africa. Although SIV asymptomatically infects many NHPs present in zoo collections, such as mandrills (Mandrillus sphinx), drills (Mandrillus leucophaeus), De Brazza’s monkeys (Cercopithecus neglectus), mangabeys, and talapoin monkeys (Miopithecus talapoin), experimental infection of macaques with SIV or the genetically engineered SHIV recombinants may result in a clinical immunodeficiency disease indistinguishable from human AIDS. Therefore, persons working with SIV-infected or SHIV-infected primates have increased risk of exposure to these lentiviruses with unknown health consequences.
To investigate the possible exposure of workers to SIV, a study conducted by the U.S. Centers for Disease Control and Prevention (CDC) tested more than 3000 samples from humans with occupational exposure to NHPs using HIV-2 serologic assays.13,20 Two samples (0.06%) were positive for antibodies cross-reactive to SIV, although the sample pool included an unknown number of repeated tests for some participants; therefore the actual prevalence may be slightly higher. One sample was associated with a laboratory worker previously identified to be infected with SIV who reported handling SIV-infected primate samples and SIV-infected culture material without wearing gloves and while having severe dermatitis of the hands and forearms. The worker remained seropositive for SIV since shortly after the exposure occurred without increases in antibody titer. SIV sequences were detected in this person at two time points surrounding the isolation of SIV (SIVhu) from this individual’s PBMCs 2 years after the exposure. The second worker, also identified previously, had remained persistently seropositive for antibodies to HIV-2/SIV for approximately 11 years after a needlestick exposure with SIV-infected macaque blood.18 A third person with antibodies to SIV had seroreactivity to SIV disappear shortly after a needlestick accident involving an SIV-infected macaque. Evidence of SIV infection in zoo workers has not been reported.13,20
These results suggest that primary cross-species transmission of lentiviruses may not always result in associated pathology, although additional clinical follow-up of these persons may be necessary to evaluate diseases with periods of long clinical latency. Similarly, “endpoint” infections have been suggested for HIV-2 subtypes C, D, E, F, and G, which, like subtypes A and B, are believed to be the result of cross-species transmission of SIV from sooty mangabeys.1
TYPE D SIMIAN RETROVIRUS (SRV)
Epizootiology
A simple retrovirus and an oncovirus, SRV may be prevalent up to 90% in some populations of wild and captive macaques and includes five different serotypes (types 1-5).10 Serotypes 1 and 3 are found mostly in rhesus macaques (Macaca mulatta), serotype 2 is found in pig-tailed (M. nemestrina) and cynomolgus (M. fascicularis) macaques, serotype 4 has only been isolated from a cynomolgus macaque, and serotype 5 was found in rhesus macaques imported from China. Serotype 3 is also known as the Mason-Pfizer monkeyvirus (MPMV).10 In addition to macaques, SRV has been isolated from squirrel monkeys (Saimiri sciureus), spectacled langurs (Trachypithecus obscurus), and yellow baboons (Papio cynocephalus). All three isolates were determined to be endogenous retroviruses that are found in the germline, thus are present in every host cell, and are recognized as self; therefore, endogenous retroviruses usually do not trigger an immune response and present a seronegative status. A new SRV, designated type 6, has been reported recently in the PBMCs of an Indian langur (Semnopithecus entellus), but SRV antibodies and multiple tissues were not tested in this animal to confirm this was also an endogenous retrovirus. Antibodies for SRV have been reported in wild captured talapoin monkeys (Miopithecus talapoin), suggesting that this virus may be endemic in primates from West Africa.13
Endogenous retroviruses are typically not highly transmissible horizontally. The virus has been isolated from blood, saliva, urine, and other body fluids, and thus SRV is transmitted through sexual contact, bite wounds, and from dam to infant, both transplacentally and postnatally.10 Latent infections may occur, and apparently healthy carrier animals have been recognized, particularly in cynomolgus macaques (Macaca fascicularis). These animals remain clinically asymptomatic but may shed the virus either continuously or intermittently for long periods before simian acquired immunodeficiency syndrome (SAIDS) eventually develops. Asymptomatic virus-positive animals may be antibody negative, making their identification by serology alone difficult.
Expression of Clinical Disease
As the etiologic agent of SAIDS, SRV was associated with outbreaks occurring in the 1980s in many U.S. primate centers. This syndrome has been associated with opportunistic infections, cutaneous and retroperitoneal fibromatosis, necrotizing stomatitis with osteomyelitis (NOMA), acute death, fever, anemia, neutropenia, lymphopenia, thrombocytopenia, hypoproteinemia, persistent diarrhea, lymphadenopathy, splenomegaly, weight loss, thymic atrophy, and fibroproliferative disorders. Disease has been associated only with macaques and may be sporadic in individually housed chronic carrier animals, enzootic in large breeding groups with positive animals, or epizootic with high mortality rates, after virus introduction to a group of naive animals.10
Interpretation of Diagnostic Assays
Because of these inapparent carrier states and the extremely high mortality rates in some naive macaque populations, adequate testing of both long-term collection animals and newly acquired animals is essential to prevent spread of the virus. Both serologic screening and virologic screening by culture or PCR testing of PBMCs (or both) are needed to detect potentially healthy, virus-positive, but seronegative animals.10 Seropositive animals that have recovered from clinical disease, but are latently infected and thus negative by viral isolation, may undergo recrudescence and shed virus later.11 Criteria for WB positivity included reactivity to at least one Gag protein (p24, p27) and at least one Env protein (gp20, gp70). Sera showing no reactivity to these antigens are considered negative, whereas sera showing reactivity to a single viral protein are considered seroindeterminate. All nonnegative (i.e., positive and indeterminate) sera are further tested in an indirect immunofluorescence assay (IFA) to provide serologic resolution. It has been suggested that PBMCs may not be the optimal tissue to analyze for detection of latent SRV infections, and that SRV proviral DNA may be more readily detected in bone marrow and other tissues from infected seropositive macaques whose PBMCs are repeatedly virus negative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree