O
Ophthalmic Examination
PREPARATION: IMPORTANT CHECKPOINTS
• Adequate restraint will greatly aid examination and is generally best performed by an assistant, not the owner. Muzzle dogs if necessary.
• Examine the animal at eye level (on exam table); large dogs may be examined on the floor, sitting up, and if cooperative, backed into a corner.
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO AVOID
• Fragile eyes (ruptured eyes, descemetoceles) should be handled extremely gently if at all. Note: Do not palpate, retropulse, or measure intraocular pressure in these eyes.
PROCEDURE
• Grossly assess functional vision as the animal walks to the exam room: does the animal show signs of adequate vision, such as confidently maneuvering and successfully avoiding objects? Or does it walk slowly and cautiously with head down, using its whiskers and other senses to navigate? Is it easily frightened by obstacles? Does vision appear to be worse on one side than the other?
• Looking at the face head-on in normal room light, assess the overall appearance and symmetry of the head and orbits; resting pupil size; and globe size, position, and motility. Note any ocular discharge or tear staining:
○ Palpate the muscles of mastication, and assess the degree of globe retropulsion through the closed lids. Palpate both eyes simultaneously to best assess symmetry.
• Assess the cranial nerves bilaterally (see p. 1311):
○ Menace response (II afferent, VII efferent): covering one eye, perform a menacing gesture to the open eye to elicit a blink, without stimulating the vibrissae (whiskers) or face. Hold your hand flat and vertical with its lateral aspect toward the animal’s eye to allow testing of different areas of the visual field while minimizing air motion:
▪ Note that diffuse cerebellar disease may cause absence of the menace response without loss of vision.
○ Palpebral reflex (sensory V afferent, VII efferent): lightly touch the lateral and medial canthi to stimulate blinking. Observe for completeness of blinking:
○ Dazzle reflex (II afferent, VII efferent): rapidly apply a very bright focal light to one eye. This should cause the eye to blink (subcortical pathway). An intact dazzle does not alone signify vision so much as some degree of retinal and optic nerve function.
• If significant mucoid ocular discharge is present, measure tear production:
○ Gently wipe away very large clumps of discharge without touching the lid margins or ocular surface.
○ Keeping the proximal end of the STT strip in the package, fold at the notch. Place the folded tip between the lower lid and globe. Close lids if necessary; keep strip in for 60 seconds, and immediately replace if blinked out.
• If corneal ulceration is suspected, apply fluorescein. Place a drop of sterile saline on a fluorescein strip, and lightly touch the strip to the bulbar conjunctiva. Allow the lids to blink. Flush excess fluorescein with saline to prevent false-positive results. Examine in a darkened room, ideally with cobalt light/Wood’s lamp:
• Carefully examine the surface and anterior segments of the eyes in dim light with the transilluminator. Note the lid margins, nasolacrimal puncta, bulbar and palpebral conjunctiva, cornea and anterior chamber, iris surface, pupil, and anterior lens:
• Examine the fundus, noting the optic nerve, retinal vessels, tapetum, and nontapetum:
○ A drop of 1% tropicamide may be given for mydriasis if there is no suspicion of glaucoma. Wait 20-30 minutes after applying; the effects will last for 2-4 hours.
○ Indirect ophthalmoscopy: the preferred method, as it provides a wider view that is easier to interpret, allows three-dimensional visualization, and is safer for the examiner:
▪ Using a fundic lens and transilluminator, position yourself as for performing retroillumination (as described above) an arm’s length away from the animal. The assistant should hold the lids open. If using an aspheric lens, hold the lens’ flatter side (white rim) toward the animal. Place the lens 1-2 inches from the animal’s eye, and then move it toward you slightly until the fundic view appears (inverted and backwards) to fill the lens. Examine the whole fundus, adjusting as if the “fulcrum” of the line of sight is at the animal’s lens (e.g., move laterally for a more medial view).
○ Direct ophthalmoscopy: provides a highly magnified view of very small areas of the fundus:
▪ To allow the widest area to be examined, use your right eye to examine the animal’s right eye, and use your left eye to examine the animal’s left eye.
▪ With the largest white circle setting and the diopter dial set on 0, focus on the fundus by viewing the eye from 2-6 inches (5-15 cm) from the animal. View as many areas as possible, adjusting the focus if needed by changing diopter settings. These lenses allow for focusing at different depths (for a fixed distance from the animal) and also can adjust for any correction required by your eyes. The view through the direct ophthalmoscope is a direct image (not reversed).
▪ If the light causes blepharospasm or resistance on the part of the animal, dim the light, apply the polarizing filter, or use a smaller circle size.
▪ The slit beam (thin rectangle) setting may help in detecting three-dimensionality and depth in anterior segment or fundic lesions.
▪ The crosshairs in the fixation aperture may be used for measuring very small retinal lesions. Size comparison may also be made to the size of the optic nerve head (optic disc).
▪ The red-free (green) filter allows for differentiation between fundic blood, which appears black, and melanin, which appears brown.
• If the eye is red or cloudy, the pupil is abnormal, or there is vision loss, perform tonometry. Note that “digital tonometry” (mere globe palpation with the fingers) is inadequate to measure intraocular pressure. Apply a drop of topical anesthetic (may not be necessary with TonoVet). Avoid pressure on the ventral neck.
○ Schiøtz: assemble the tonometer and clean the footplate. Test the tonometer by placing it on the included test block (gives a scale reading of 0). Position the animal with iris plane parallel to floor (dorsal recumbency or sitting up with nose pointed to the ceiling). Holding the tonometer by the handles and keeping it vertical, gently rest the footplate on the central cornea (not the third eyelid or sclera) until a single reading is produced. Repeat two to three times, and use included chart to get actual intraocular pressure (IOP). If IOP is >25 mm Hg, repeat with next additional weight. Record IOP in mm Hg (not as scale readings). Clean tonometer after use.
○ Tono-Pen: Properly fit a clean tip cover, turn on, and calibrate if necessary. Gently touch the instrument tip to the cornea, keeping it perpendicular to the eye; it does not need to be horizontal. Repeat several times until a long beep is heard. A bar over the “5%” at the bottom indicates a statistically significant reading (mean given in mm Hg).
○ TonoVet: Properly insert a new probe tip. Turn on and ensure that proper species calibration is on. Holding the instrument horizontal with the tip 4-8 mm from the cornea, repeatedly press the button to take readings until a long beep is heard. A nonblinking “d” before the reading indicates a statistically significant, dog/cat-calibrated reading.
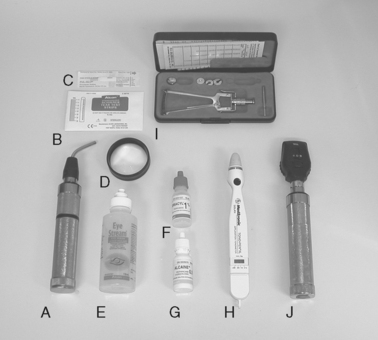 OPHTHALMIC EXAMINATION Equipment and materials that may be used during an ophthalmic examination. A, Finnoff transilluminator. B, STT strips. C, Fluorescein dye–impregnated strip. D, Lens. E, Sterile eye irrigating solution. F, 1% tropicamide solution. G, 0.5% proparacaine solution. H, Tonometer—Tono-Pen. I, Tonometer—Schiøtz. J, Direct ophthalmoscope.
OPHTHALMIC EXAMINATION Equipment and materials that may be used during an ophthalmic examination. A, Finnoff transilluminator. B, STT strips. C, Fluorescein dye–impregnated strip. D, Lens. E, Sterile eye irrigating solution. F, 1% tropicamide solution. G, 0.5% proparacaine solution. H, Tonometer—Tono-Pen. I, Tonometer—Schiøtz. J, Direct ophthalmoscope.


