Chapter 31. Nutritionally Responsive Dermatoses
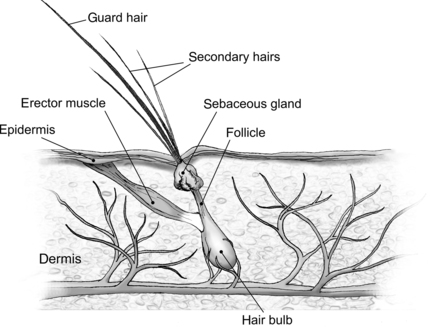
The skin (integument) is the largest organ in the body; together skin, hair, and dermis make up 24% of the body weight (BW) of a newborn puppy and 12% of the BW of an adult dog. 1 The integument is metabolically active. It provides sensory input, acts as a barrier to protect the body from physical and infectious injury, is important for normal temperature control and immunoregulation, and serves as a reservoir for certain nutrients. The health of a pet’s skin and hair coat can be affected by a variety of nutrients, most importantly protein, vitamin A, vitamin E, the essential fatty acids (EFAs), and zinc (Figure 31-1). Dogs and cats that are consuming high-quality, complete, balanced pet foods are unlikely to suffer from a serious deficiency or excess of any of these nutrients. However, feeding a poorly formulated or stored commercial food, or preparing a homemade diet that is not correctly balanced, can contribute to skin disorders. In addition, any metabolic or functional disease that affects a pet’s ability to digest, absorb, or use nutrients can cause secondary nutrient imbalances that may manifest as dermatoses. A third way nutrition can affect the health of the skin relates to inflammatory skin disease. The development of an adverse reaction to one or more components in the diet can be the cause of inflammatory dermatoses in dogs and cats. In addition, diet can be an important component in the management of other forms of inflammatory skin disease in dogs and cats such as atopic dermatitis and feline miliary dermatitis.
PROTEIN AND SKIN HEALTH
The importance of protein for the maintenance and development of skin and coat health is well documented. Hair is more than 90% protein and contains high amounts of the sulfur amino acids, methionine and cystine. Normal turnover of skin cells and keratinization also impose a demand for protein. Together, skin and coat needs account for up to 30% of an animal’s daily protein requirement. 2 Although uncommon, protein deficiency in dogs and cats can be seen in association with starvation, as a result of disease-induced anorexia, or in response to prolonged feeding of an inadequate diet. Changes to the skin and hair coat that develop during protein deficiency include abnormal keratinization, depigmentation of hair shafts, and changes in sebaceous and epidermal lipids. Coat hairs become brittle and break off easily, and coat growth slows or stops. The lipid layer of the epidermis becomes abnormal and loses its function as a protective barrier. The skin becomes scaly, greasy, and susceptible to secondary bacterial infections. When healthy pets are fed balanced, complete pet foods, signs of protein deficiency are highly unlikely.
Dietary protein may also affect skin health in terms of hypersensitivity response and effects upon subcutaneous lipid metabolism. The importance of dietary protein for pets with adverse food reactions is discussed later in this chapter (pp. 396-402). It has also been theorized that feeding different types of proteins may affect skin and serum lipid concentrations in dogs. For example, plant proteins have been reported to be hypocholesterolemic compared with animal proteins, when fed to monkeys and rabbits. 3. and 4. In a study with dogs, six different protein sources (chicken, pork, lamb, fish, beef, and soy) were sequentially fed to a group of 12 dogs. 5 No differences were observed between protein treatment groups in skin histology, signs of inflammation or pruritus, or skin fatty acid levels. Several of the dogs fed pork showed an increase in scale production and a decrease in hair regrowth following skin biopsies, suggesting that pork should be avoided as a protein source in dogs with seborrhea. However, additional research is needed to further investigate the role of pork in skin health in dogs and cats.
Another study compared the effects of feeding soy protein versus a meat-based protein source and soy oil versus poultry fat on serum cholesterol, serum lipids, and cutaneous fatty acid concentrations in dogs. 6 Dietary protein had no effect upon serum cholesterol concentration and only marginal effects upon serum and skin fatty acid concentrations. However, dogs fed soy oil–containing diets had higher linoleic acid (LA) and lower oleic acid concentrations in skin and serum when compared with dogs fed diets containing poultry fat. Dogs fed diets containing poultry fat also had higher concentrations of serum arachidonic acid (AA). An important finding of this study is that while the type of fat significantly influenced serum and skin fatty acid and cholesterol concentrations in this study, the protein source did not affect lipid profiles.
The results of these two studies indicate that protein source does not significantly affect changes in fatty acid values or skin architecture in the dog. The effects of dietary protein on inflammatory skin disease in dogs are more related to allergenicity and frequency of exposure rather than to an influence upon fatty acid metabolism or homeostasis (see pp. 397-398). While it is possible that fat content in various protein sources may affect cutaneous and plasma fatty acid concentrations, such effects are associated with the protein’s fatty acid composition and not with protein characteristics per se.
VITAMIN A–RESPONSIVE DERMATOSES
Vitamin A is necessary for normal epithelial cell differentiation and maintenance and for the process of keratinization (see Section 1, pp. 27-29). Both deficiencies and excesses of this vitamin cause skin lesions in dogs and cats. Signs include hair loss and poor coat condition, hyperkeratinization of the epidermis and hair follicles, scaling of the skin, and an increased susceptibility to secondary bacterial infections of the skin. 1 Vitamin A toxicity is most commonly caused by feeding an all-liver diet or by oversupplementation with cod liver oil. Nutritional deficiencies of vitamin A (retinol) are uncommon and are accompanied by visual and digestive problems. 2
More common than deficiencies or excesses, however, are certain types of skin disorders that are responsive to treatment with supplemental vitamin A or retinoids (natural and synthetic vitamin A analogs). The administration of vitamin A and the retinoids appear’s to have both physiological and pharmacological effects. These compounds have been used successfully in humans and animals to treat cases of idiopathic seborrhea that are not caused by a vitamin A deficiency. 7. and 8. Seborrhea is a general term that describes the overproduction of oils and other protective secretions by the sebaceous glands in the skin. The skin usually becomes flaky, greasy, or both. Because the epidermal lipid layer is abnormal, the animal becomes prone to secondary bacterial skin infections that can cause pruritus and further damage to the skin. Treatment of seborrhea in companion animals is usually directed toward determining the underlying cause and correcting it. However, in a substantial number of cases, an underlying mechanism cannot be identified and treatment is directed primarily toward the relief of clinical signs.
Certain types of seborrhea in dogs and cats respond favorably to vitamin A. The most common form is idiopathic seborrhea in Cocker Spaniels, which can often be kept in complete remission with vitamin A therapy. 9.10. and 11. The disorder and response to vitamin A therapy has also been reported in Labrador Retrievers and Miniature Schnauzers. 10. and 12. Vitamin A–responsive seborrhea is characterized by dry and scaly skin that progresses to oily changes. Affected dogs eventually develop large, hyperkeratotic plaques (composed of sebum and keratin) and marked follicular plugging. Lesions are most prominent on the underside of the thorax and abdomen. Hair loss and skin changes are accompanied by secondary bacterial folliculitis. Pruritus and inflammation may or may not be present. Almost all reported cases also show moderate to severe otitis externa.
Cases of vitamin A–responsive seborrhea usually do not respond to the traditional treatments for seborrhea, which include medicated shampoos, antibiotic therapy, and glucocorticoid therapy. Although clinical signs can be used in support of a diagnosis, the diagnosis of vitamin A–responsive seborrhea can only be confirmed through favorable response to supplementation. A dose of 10,000 international units (IUs) per day is suggested, although levels as high as 50,000 IUs/day have been used. 9.10.11. and 12. A decrease in clinical signs is usually seen within 4 weeks, with complete remission within 2 to 6 months of starting treatment. Attempts to reduce the level of vitamin A, or to withdraw therapy, result in a relapse of clinical signs, indicating that lifelong therapy is necessary. The dosages used represent 6 to 10 times the dog’s normal requirement for vitamin A. However, no signs of vitamin A toxicity have been observed in the reported cases, even after several years of therapy. Other studies have indicated that much higher levels of vitamin A are necessary to induce clinical signs of vitamin A toxicity in dogs. 13. and 14.
A second skin disorder that has been shown to be responsive to vitamin A supplementation is sebaceous adenitis. This chronic skin disease is characterized by the development of lymphocytic, granulomatous, or pyogranulomatous inflammation of the sebaceous glands, resulting in scaling, skin lesions, and hair loss. Over time, sebaceous glands are progressively destroyed, and inflammation diminishes. Sebaceous adenitis is genetically influenced. Standard Poodles, Akitas, Chow Chows, and Vizslas are more frequently affected with this disorder than the general population of dogs. 15. and 16. In Poodles, the disease is believed to be transmitted by an autosomal recessive gene. 17 Some of the treatments used to manage sebaceous adenitis include antiseborrheic shampoos, topical application of propylene glycol or EFAs, and systemic administration of cyclosporine or synthetic retinoids (vitamin A derivatives).
A study of 30 dogs examined the efficacy of using two synthetic retinoids, isotretinoin and etretinate, to treat the clinical signs of sebaceous adenitis. 18 Dogs that had been diagnosed with the disorder were treated for a minimum of 2 months with one of the two retinoids. Forty-seven percent of the dogs given isotretinoin and 53% of the dogs given etretinate were successfully treated and were maintained on retinoid therapy indefinitely. Although it was previously thought that Akitas respond poorly to synthetic retinoids, this study reported successful treatment in 10 of the 11 Akitas included in the study. 18. and 19. These results suggest that retinoids may be an effective treatment for some dogs with sebaceous adenitis. An initial dosage of 1 milligram (mg)/kilogram (kg) of BW per day of either isotretinoin or etretinate is recommended.
It is important to note that these skin conditions are not caused by a vitamin A deficiency. In all reported cases, the dogs were being fed a high-quality, complete and balanced, commercial dog food. Moreover, serum levels of vitamin A were normal, and no other signs of vitamin A deficiency were observed. One group of investigators also reported that the skin changes seen in cases of vitamin A–responsive dermatosis differed significantly from those seen with a true vitamin A deficiency. 9 It is likely that the effect of vitamin A is the result of a pharmacological action of the vitamin on epithelial cells, rather than a result of the vitamin’s role as an essential nutrient. 7
Some cutaneous disorders that respond to vitamin A therapy are not actually caused by a vitamin A deficiency. In these cases vitamin A therapy is required throughout the pet’s life, but therapeutic dosages have not been found to be toxic. It is believed that there is a genetic basis for vitamin A–responsive seborrhea in Cocker Spaniels and for sebaceous adenitis in Standard Poodles, Akitas, Chow Chows, and Viszlas.
VITAMIN E–RESPONSIVE DERMATOSES
The term vitamin E refers to a group of compounds called the tocopherols that act as biological and food antioxidants. Alpha-tocopherol is the most biologically active form of vitamin E. Within the body, vitamin E functions as a free-radical scavenger, protecting cell membranes and tissues from oxidative stress. An animal’s dietary requirement for vitamin E is dependent upon dietary intake of polyunsaturated fatty acids (PUFAs), with an increase in requirement occurring when high levels of PUFAs are fed (see Section 1, p. 31 and Section 2, p. 110). Vitamin E deficiency is uncommon in dogs, but has been reported in cats fed high-fat diets and results in a pathological condition called pansteatitis (see Chapter 26, p. 279 for a complete discussion). Although a poor hair coat may be seen in cats with pansteatitis, the primary manifestations of vitamin E deficiency are related to the necrosis and inflammatory changes in subcutaneous and intraabdominal fat.
Conversely, supraphysiological doses of vitamin E have some efficacy in the management of a skin condition called primary acanthosis nigricans in Dachshunds. This disorder is characterized by hair loss and extreme hyperpigmentation (blackening) and thickening of the skin. As the disease progresses, varying degrees of greasiness, crusting, rancid odor, and secondary bacterial infections develop. Pruritus is usually absent or mild during the early stages of the disorder, but it may become more pronounced as secondary infections occur. In one study, a group of eight Dachshunds with acanthosis nigricans were given 200 IUs of alpha-tocopherol daily. 20 All eight dogs showed improvement within 60 days. Skin inflammation, crusting, and pruritus completely subsided, although hyperpigmentation did not improve. Clinical signs did not reappear in any of the dogs within follow-up periods of 7 months to 3 years. All of the dogs were maintained on the vitamin E supplementation throughout this time. None of the owners attempted to decrease the dose or withdraw the supplementation, so it is not known if long-term vitamin E supplementation is necessary in all cases.
A medium-sized (20-kg) adult dog has a minimum daily requirement for vitamin E of about 10 IUs. Most commercial pet foods will supply a 20-kg dog with between 20 and 50 IUs/day. The levels that were fed in this study represent 4 to 10 times the dog’s normal daily intake of vitamin E, but no toxicity signs were observed in any of the eight dogs. The authors concluded that vitamin E may offer a therapeutic alternative for some cases of primary canine acanthosis nigricans. In all cases, the disorder is chronic and persistent, and therapy is directed toward control rather than cure. Many dogs with acanthosis nigricans respond favorably to systemic treatment with corticosteroids. However, concern over the immediate and long-term side effects of corticosteroid therapy dictates the need to find alternative or adjunctive treatments, one of which may be supplemental vitamin E.
Vitamin E therapy has also been used with varying levels of success in dogs with discoid lupus erythematosus and dermatomyositis. 21. and 22. However, not all dogs with these disorders respond to vitamin E therapy, and additional research needs to be conducted to determine an effective dose range of vitamin E. Conversely, vitamin E supplementation has been shown to be ineffective as an antiinflammatory and antipruritic agent for the treatment of atopic dermatitis (allergic dermatitis) in dogs (Table 31-1).
| D isorder | B reeds | S uccess of vitamin E supplementation |
|---|---|---|
| Demodicosis | All | Variable |
| Primary acanthosis nigricans | Dachshunds | Yes |
| Discoid lupus erythematosus | All | Variable |
| Dermatomyositis | Collies, Shetland Sheepdogs | Variable |
ZINC-RESPONSIVE DERMATOSES
The essential mineral zinc has several functions that affect skin health and coat quality. As an integral component of several metalloenzymes and a cofactor for both ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) polymerases, zinc plays an important role in regulating cellular metabolism and cell division. Zinc is also needed for the synthesis of fatty acids and is essential for immune system health and normal inflammatory responses. In adult animals, a deficiency of zinc manifests principally as changes to skin and coat health. In young animals, skin changes are accompanied by impaired growth, inappetance, and other health problems.
Dermatoses that respond to zinc supplementation occur in companion animals for a number of reasons. Genetic disorders are responsible for impaired zinc absorption and metabolism in several breeds of dogs. 23 A defect in the mucosal transfer system for zinc in the small intestine occurs in Alaskan Malamutes, Siberian Huskies, and American Eskimos. Bull Terriers with lethal acrodermatitis also have an inability to absorb dietary zinc (see Chapter 27, pp. 299-300 for a complete discussion). Zinc deficiency can also result from inadequate levels of the mineral in the diet and from the presence of other nutrients or components that interfere with zinc absorption. 24. and 25. Specifically, increased levels of calcium and phytate will interfere with the bioavailability of dietary zinc and can lead to zinc deficiency. 26 Naturally occurring cases of zinc-responsive dermatoses are rare in dogs and have not been reported in cats. The reported cases in dogs have all been associated with feeding foods that contain either marginal levels of zinc, cereal-based foods containing high levels of phytate, poor-quality foods containing high amounts of calcium, or a combination of these factors. Growing dogs are most susceptible to zinc deficiency, and excessive supplementation with calcium during growth also has the potential to result in dermatoses associated with zinc deficiency. 25. and 27.
In dogs, cases of diet-induced zinc deficiency are associated with feeding foods that contain either marginal levels of zinc, cereal-based foods that contain high levels of phytate, poor quality foods that contain high amounts of calcium, or a combination of these factors. Growing dogs are most susceptible to zinc deficiency, and excessive supplementation with calcium during growth also has the potential to result in dermatoses associated with zinc deficiency.
The skin lesions of zinc-responsive dermatosis are seen on the face, over pressure points, and on the foot pads (Figure 31-2). 28. and 29. In extreme cases, the lesions eventually spread over the entire body. Affected areas are characterized by hair loss, redness, inflammation, crusting, and pruritus. Secondary skin infections are also seen. Diagnosis is usually made by diet history, breed, physical examination, and skin biopsy. Positive response to oral zinc supplementation without changing the diet can be used to confirm a diagnosis. 28 However, because dogs vary considerably in response and because a wide range of effective dosages has been reported, it is difficult to make recommendations for appropriate dosages of zinc to use. In addition, zinc requirements and response to supplementation are influenced by the animal’s physiological state, the presence of inhibitory components in the diet, and the form of zinc that is used. 30 A dose of 10 mg of zinc sulfate/kg per day or 1.7 mg of zinc methionine/kg per day is recommended by some authors. 22. and 28. The smaller dose of zinc methionine was suggested because of presumed increased bioavailability of the organic form. However, increased bioavailability of zinc methionine appears to be significant only when there is high metabolic demand such as during growth or when there are inhibitory components in the food. 31. and 32. Other authors suggest an initial dosage that provides 2 to 3 mg/kg BW of elemental zinc/day. 23 The initial dose should be administered for at least a 30-day period to determine the response to treatment. A response should be seen within 6 weeks of initiation of supplementation and, in most cases, skin lesions show a rapid response, with complete healing within 2 weeks.
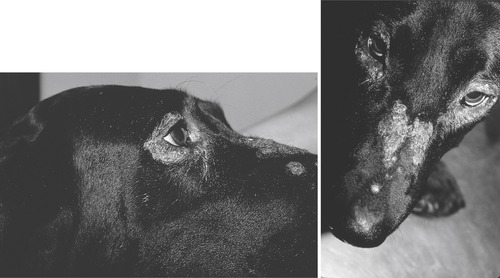 |
| Figure 31-2 (Courtesy Candace Sousa, DVM, Animal Dermatology Clinic, Sacramento, Calif.) |
Zinc supplementation confirms a diagnosis of zinc-responsive dermatosis and aids in rapid recovery. In cases that occurred as a result of an inadequate diet (i.e., presence of inhibitory substances) or excessive supplementation, feeding a high quality complete and balanced food that supplies adequate levels of zinc is recommended. Additionally, there is some evidence that increasing EFAs may have a synergistic effect with zinc in some animals. 23. and 33. In other species, supplementation with EFAs has been shown to ameliorate the clinical effects of zinc deficiency. 34 This may occur because clinical signs of EFA and zinc deficiencies are similar. The presence a deficiency in both nutrients negatively affects sebum production and skin health. It is possible that a subclinical EFA deficiency occurred in dogs that reportedly showed an additive response to zinc and EFA (LA). This would be expected if the underlying cause of zinc deficiency was a poor diet. Alternatively, fat absorption may be reduced in dogs with zinc-responsive dermatoses, which could lead to a subclinical deficiency state. 35 Continued supplementation with zinc after correction of the diet is necessary when an inherited problem with zinc metabolism exists.
ESSENTIAL FATTY ACIDS AND SKIN DISEASE
As components of cell-membrane phospholipids and precursors for a variety of regulatory compounds, the EFAs maintain the health and integrity of epithelial tissue in the body. The omega-6 fatty acids that are considered to be essential nutrients include LA in dogs and LA and AA in cats (see Section 2, pp. 81-83). Although the essential nature of omega-3 fatty acids has been controversial, alpha-linolenic acid (ALA) is considered by most nutritionists to be conditionally essential for dogs and cats. 36 LA is specifically responsible for maintaining the cutaneous water permeability barrier, while AA, as a prostaglandin precursor, is needed for normal epidermal proliferation. Other nutrients that may be important to support a healthy epidermal barrier in dogs include pantothenate, choline, nicotinamide, histidine, and inositol. 37 The long-chain omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), support normal cell membrane fluidity and also have antiinflammatory and immunostimulatory properties. Because of these functions and the skin’s rapid cell turnover rate, the skin is especially susceptible to EFA deficiencies. 38
In dogs and cats, signs of EFA deficiency become apparent within 2 to 3 months of consuming a deficient diet. Initial signs include a reduction in surface lipid production, which results in a dry, dull coat; hair loss; and the eventual development of skin lesions. Over time, the skin becomes greasy, pruritic, and susceptible to infection. A change in the surface lipids in the skin compromises the permeability layer, alters the normal bacterial flora, and predisposes the animal to secondary bacterial infections. 2 Epidermal peeling, interdigital exudation, and otitis externa have also been reported in EFA-deficient dogs. When EFA deficiency is uncomplicated by other nutrient imbalances, skin lesions show a response to dietary correction within 1 to 2 months. 2
Naturally occurring skin disease as a result of EFA deficiency is rare in dogs and cats today. Healthy companion animals that are fed high-quality foods are not at risk of developing an EFA deficiency. When a deficiency does occur, it is usually the result of feeding a food that is either poorly formulated or has been stored improperly. If the food has been stored at high temperatures or beyond the stated expiration date, there is a risk of EFA loss as a result of oxidative damage to the food. When an EFA deficiency is suspected, it is better to change the diet to a food that is well formulated and has been stored properly, rather than to attempt to correct a deficiency by adding supplemental fatty acids.
Therapeutic Role of Omega-6 and Omega-3 Fatty Acids
EFA supplementation and dietary manipulation of EFA metabolism appear to have some efficacy in the treatment of certain skin disorders that are not the result of a dietary EFA deficiency. The PUFAs are divided into several series based on the position of the first double bond in the carbon chain. Of greatest interest are the omega-3 and omega-6 series of fatty acids. The omega-3 (or n-3) fatty acids have the first double bond located at the third carbon atom from the terminal methyl group. The omega-6 fatty acids have the first double bond at the sixth carbon atom (see Section 1, p. 19). The effects of both types of fatty acids upon cell membrane fluidity and permeability, and their availability for synthesis of new compounds, are affected by the molecule’s chain length, the degree of saturation, and the position of the first double bond.
Algae synthesize large amounts of omega-3 fatty acids. As a result, most marine animal tissues contain high concentrations of the omega-3 PUFAs. Sources of omega-3 fatty acids in pet foods include cold-water fish oils as well as whole-fat flax (flax oil is an enriched source of ALA). Land animals, in contrast, have higher concentrations of the omega-6 fatty acids in their tissues because most plants consumed by these animals contain greater amounts of omega-6 than omega-3 fatty acids. 39 Enriched sources of omega-6 fatty acids in pet foods include corn, safflower, sunflower, and cottonseed oils. Soy and canola oils contain high levels of omega-6 fatty acids, as well as some ALA. Another omega-6 fatty acid, gamma-linolenic acid (18:3n-6) (GLA), is found in borage, black currant, and evening primrose oil. 40 Oils containing a large proportion of monounsaturated fatty acids, such as olive oil, and saturated animal fats are not considered enriched in either omega-3 or omega-6 fatty acids (Table 31-2).
| Note: The following fats and oils are poor sources of omega-6 and omega-3 fatty acids: lard, mutton fat, coconut oil, and olive oil. | |
| EPA, Eicosapentaenoic acid (20:5n-3). | |
| O mega-6 fatty acid sources | O mega-3 fatty acid sources |
|---|---|
| Corn oil (70% linoleic acid) | Coldwater fish oils (12%-15% EPA) |
| Safflower oil (78% linoleic acid) | Flaxseed (57% alpha-linolenic acid) |
| Sunflower oil (69% linoleic acid) | Canola oil (8% alpha-linolenic acid) |
| Cottonseed oil (54% linoleic acid) | Soybean oil (7% alpha-linolenic acid) |
| Soybean oil (54% linoleic acid) | |
Fatty Acids as Eicosanoid Precursors
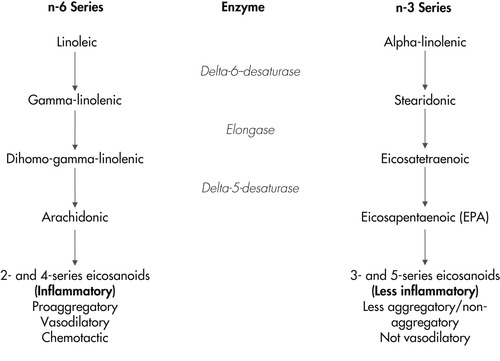
In addition to providing structural integrity and fluidity to cell membranes, membrane fatty acids also have specific roles in the regulation of cell functions. The PUFAs AA, GLA, EPA, and DHA are all precursors for the synthesis of eicosanoids. Eicosanoids are immunoregulatory molecules that have local and short-lived hormonelike effects. The four primary types of eicosanoids are prostaglandins, leukotrienes, prostacyclins, and thromboxanes. Eicosanoids are involved in inflammatory reactions, immunoregulation, and epidermal cell proliferation. When cellular injury occurs, membranes release their component fatty acids, which are then metabolized to eicosanoids. The amount and type of eicosanoid synthesized is determined by the availability and type of fatty acid precursor from the cell membrane and by the activities of the two metabolic enzyme systems, cyclooxygenase and lipoxygenase. The omega-3 and omega-6 fatty acids produce different families of eicosanoids and also compete for these two metabolic pathways. 41
During an inflammatory response, the release and metabolism of omega-6 fatty acids produces the 2-series prostaglandins, the 4-series leukotrienes, hydroxyeicosatetraenoic acid, and thromboxane A 2 (Figure 31-3). These agents are immunosuppressive at high levels, are proinflammatory, promote platelet aggregation, and act as potent mediators of inflammation in type-I hypersensitivity reactions. 42. and 43. In contrast, the release and metabolism of omega-3 fatty acids (specifically, EPA) produces mediators with much less inflammatory activity. Those compounds are antiaggregatory, not immunosuppressive at levels normally found in the body, and vasodilatory. They include the 3-series prostaglandins, the 5-series leukotrienes, hydroxyeicosapentaenoic acid, and thromboxane A 3. For example leukotriene B 5 (LTB 5), produced from EPA, is approximately 10-fold less potent as a neutrophil chemoattractant than leukotriene B 4 (LTB 4), which is produced from AA. 44 In addition to being less biologically active than LTB 4, there is evidence that LTB 5 may inhibit the action of LTB 4, possibly via competition for cell membrane receptors. 45. and 46. These data suggest that the ratio of leukotrienes produced from AA to leukotrienes produced from EPA may be more important than absolute amounts of these compounds.
Influence of Dietary Omega-6 and Omega-3 Fatty Acids on Eicosanoid Production
Research studies with humans, laboratory animals, and companion animals have shown that levels of omega-6 and omega-3 fatty acids present in tissue cell membranes can be manipulated by diet, and that these manipulations influence the inflammatory response. Increasing the amount of omega-3 fatty acids in skin and other tissues leads to decreased production and activity of the proinflammatory eicosanoids and increased synthesis of the less-inflammatory metabolites. 47 Several factors are responsible for this effect. First, as discussed previously, omega-6 and omega-3 fatty acids compete directly for the same enzyme systems. Therefore increasing the amount of available omega-3 fatty acids competitively inhibits the metabolism of omega-6 fatty acids when fatty acids are released from membranes during an inflammatory reaction. Second, the compounds produced from the metabolism of omega-3 fatty acids are less inflammatory than those produced from AA. 48 Finally, two of the end products of EPA metabolism are LTB 5 and 15-hydroxyeicosapentaenoic acid. There is evidence that these compounds inhibit the potent proinflammatory action of omega-6–derived leukotrienes.
The amount and type of omega-3 and omega-6 fatty acids and the ratio of these two classes of fatty acids in the diet must be considered when determining effects on plasma and tissue levels and consequent eicosanoid synthesis. 49.50. and 51. For example, an early study with healthy dogs showed that feeding a diet containing an omega-6 to omega-3 ratio between 5:1 and 10:1 resulted in the production of significantly lower levels of LTB 4 and significantly higher levels of the less inflammatory metabolite LTB 5 in the skin, when compared with levels that were produced when the dogs were fed a diet with ratios of 28:1 or higher. 52 Similarly, the neutrophils of dogs fed diets containing the lowest omega-6 to omega-3 ratios (5:1 and 10:1) had decreased LTB 4 and increased LTB 5 concentrations (Figure 31-4). Cells from lipopolysaccharide-stimulated (LPS) skin biopsy samples synthesized 48% to 62% less LTB 4 and 48% to 79% more LTB 5 when compared with samples obtained prior to feeding the experimental diets. Similar changes were observed in neutrophils. These responses were considered to be clinically significant because decreases in tissue LTB 4 concentrations of 50% or more are typically large enough to attenuate an inflammatory response.
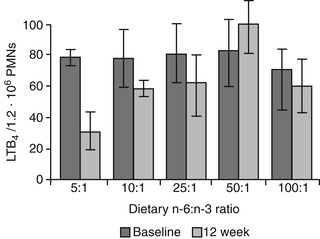 |
| Figure 31-4 (From Reinhart GA, Davenport GM: Omega-3 fatty acids and inflammation management. In Proceedings of fourth world congress of veterinary dermatology, 2000, p 32.) |
Additional research corroborated these results and further refined understanding of the specific types and amounts of omega-3 fatty acids that can modulate the inflammatory response. Healthy elderly Beagles fed diets that were supplemented with fish oil to achieve an omega-6 to omega-3 fatty acid ratio of 1.4:1 had altered plasma fatty acids (increased DHA and EPA and decreased AA) and had reduced prostaglandin E 2 (PGE 2) production by peripheral blood mononuclear cells. 53 Fish oil–supplemented dogs also showed a reduction in response to a delayed-type hypersensitivity (DTH) skin test and a blunted response to immunization with a novel protein. 54 A follow-up study was conducted to examine the relationship between plasma fatty acid concentrations and leukotriene production by peripheral blood neutrophils in dogs fed these diets for 36 weeks. 55 Plasma concentrations of EPA and AA reflected dietary intake, and a strong and significant correlation was found between the plasma ratio of EPA:AA and the ratio of LTB 5 to LTB 4 produced by stimulated neutrophils. Specifically, plasma EPA increased, plasma AA decreased, and LTB 5 produced by stimulated neutrophils increased in dogs fed fish oil–supplemented diets. The same researchers demonstrated an interaction between dietary vitamin E intake and intake of omega-3 fatty acids, suggesting that the benefits of vitamin E on DTH response are reduced when the intake of omega-3 fatty acids is very high. 56 Together, these studies suggest that there is an optimal level of omega-3 fatty acid intake and an omega-6 to omega-3 ratio that can modify the production of proinflammatory compounds but that is not high enough to negatively impact other immune system functions or vitamin E status.
In a study, a group of 15 healthy adult dogs were fed a base diet supplemented with either sunflower oil (source of LA), fish oil (source of EPA and DHA), or fish oil plus vitamin E. 57 The fish oil–enriched diets provided 1.75 grams (g) EPA/kg diet and 2.2-g DHA/kg diet, resulting in an omega-6 to omega-3 ratio of 3.4:1. After 12 weeks of feeding the test diets, in vivo measurement of the serum of dogs fed fish oil–enriched foods showed a blunted inflammatory response to LPS when compared with the response observed in dogs fed the sunflower oil–enriched diet. These results are especially significant because this was the first report that used an in vivo assay to examine LPS-stimulated cytokine response after feeding omega-3 fatty acid enriched diets. Additional studies of the same dogs showed that increasing omega-3 fatty acids in the diet did not significantly increase plasma concentrations of lipid peroxides or cause a reduction in serum vitamin E concentrations. 58 These results indicate that the dogs did not experience increased oxidative stress in response to the altered fatty acid ratios and increased intake of EPA and DHA.
Although changing the ratio of omega-6 to omega-3 fatty acids is an effective approach to modifying the production of proinflammatory and less inflammatory compounds, there is also evidence that when an equal ratio is fed and kept constant, increasing the omega-3 dose alone influences the plasma fatty acid profile. 59 Healthy dogs were fed diets containing an omega-6 to omega-3 ratio of 1:1, with varying concentrations of each class of fatty acid, supplied by corn oil and fish oil, respectively. Plasma concentrations of omega-3 fatty acids increased with increasing total intake of omega-3 fatty acids, up to a certain level of intake, regardless of the fact that omega-6 fatty acids were also increasing. These results suggest a dose-dependent response to omega-3 intake that was independent of the ratio of the two classes of fatty acids. However, it is important to note that the diets that were fed in this study already contained an “adjusted” fatty acid ratio (1:1). It is unknown (and questionable) whether a similar response would have occurred if the ratio was more similar to that seen in many commercial foods (i.e., 15:1 or greater). Second, the omega-3 fatty acids in this study were supplied as EPA and DHA (fish oil), while the omega-6 fatty acid was supplied as primarily LA (corn oil). Therefore the dose response may reflect a selective favoring of highly unsaturated fatty acids for esterification into phospholipids when compared with their precursors. It is not known if these effects would have been observed if increased omega-6 fatty acids had been supplied as AA instead of as LA. Regardless, these results suggest that when a favorable ratio is included in the diet, increasing the source of EPA and DHA while maintaining a constant omega-6 to omega-3 ratio positively affects plasma fatty acid profiles and, presumably (although not yet reported), the production of less inflammatory eicosanoids.
Research studies show that levels of omega-6 and omega-3 fatty acids in pets’ diets influence tissue levels of these fatty acids and these modifications influence the inflammatory response. Increasing the amount of omega-3 fatty acids in skin and other tissues leads to decreased production and activity of the proinflammatory eicosanoids and increased synthesis of the less inflammatory metabolites.
Dietary Fatty Acids and Atopic Dermatitis
Dogs and cats are susceptible to a wide range of inflammatory skin diseases. In dogs, these include allergic disorders, parasitic infestations, bacterial infections, and adverse reactions to food. Cats show the same disorders with the addition of miliary dermatitis and eosinophilic granuloma complex. Inflammatory skin disorders that are associated with immunoglobulin E (IgE)-mediated type-1 hypersensitivity (allergic) responses are most likely to respond favorably to modifications of dietary fatty acid concentrations. In dogs, these include atopic dermatitis (atopy), flea-bite hypersensitivity, and food hypersensitivity (discussed below). In cats, allergic skin disease also manifests as miliary dermatitis. 60 Because flea-bite hypersensitivity is best treated via stringent flea-control programs, current research studies have focused on the use of fatty acid therapy to control atopic disease in dogs and cats.
In dogs, atopic dermatitis is considered to be the most frequently diagnosed form of allergic skin disease. 61 Many animals with atopy are sensitized to multiple environmental allergens, which may include house dust mites or dust, molds, weeds, grasses, and trees. In addition, a substantial proportion of dogs with atopic dermatitis are simultaneously affected by an adverse food reaction. 62 Recent studies suggest that dogs with atopic disease have almost four times greater risk of developing concurrent food allergy than dogs without atopy. For these reasons, differential diagnosis of allergic skin disorders must always include consideration of the presence of both atopy and adverse food reaction and the role that multiple allergens and the pruritic threshold plays in the development of clinical signs.
Atopy is characterized by pruritus, self-trauma to the skin, and secondary bacterial or yeast infection. 63. and 64. Chronic otitis externa is also a common finding. Diagnosis of atopic dermatitis in dogs typically uses the criteria of Willemse, which includes case history information and clinical signs. 65 Observations of breed and family predilections, along with results of limited breeding trials, indicate that some dogs are genetically predisposed to develop atopy. 66 Breeds identified as being at increased risk include Chinese Shar-Peis, Dalmatians, Irish Setters, Golden Retrievers, Boxers, Labrador Retrievers, Belgian Tervurens, and several Terrier and toy breeds. Cats with atopic disease often develop military dermatitis, which is characterized by small papules and crusts found most frequently on the head and neck.
Clinical signs develop when the animal is exposed to the offending antigen(s) and IgE-sensitized mast cells in the skin degranulate and release a host of inflammatory mediators. These compounds include histamine, heparin, proteolytic enzymes, chemotactic factors, and various types of eicosanoids. As discussed previously, the type and proportions of fatty acids present in the cell membranes and activity of the cyclooxygenase and lipoxygenase enzyme systems determine the specific type of eicosanoids that will be produced during an inflammatory response. In pets with atopy, the immediate-type hypersensitivity response (IgE-mediated) is followed by a late-phase response that develops 6 to 12 hours following mast cell activation. 67 In addition, some of the released cytokines function to recruit inflammatory cells to the local area, which can persist for several days and are responsible for the chronic inflammatory skin changes seen in atopic animals.
Although IgE-mediated immediate-type and delayed (late-phase) hypersensitivities are responsible for the cascade of inflammatory agents after exposure to offending allergens in most patients, it appears that other factors are also involved. 68 The most important of these is epidermal barrier function. Human subjects with atopic dermatitis have irregularities in the lipid and ceramide components of their stratus corneum (the uppermost protecting layer of the epidermis). 69 A defect in this layer can affect the skin’s function as a protective barrier, leading to increased transepidermal water loss and increased risk of penetration by allergens and infectious agents. Although these changes have not been definitely demonstrated in dogs, there is evidence that the stratum corneum of atopic dogs has structural differences when compared with normal canine skin and that these differences may involve the lipid-containing ceramides. 70 It is theorized that a stratum corneum with an impaired lipid barrier predisposes an animal to the development of hypersensitive response to contact allergens because foreign agents are able to penetrate further into the skin. Another potentially influencing factor is the presence of a defect in fatty acid metabolism. There is evidence that dogs with atopy may have compromised ability to metabolize omega-6 fatty acids due to reduced activity of delta-5 and/or delta-6 desaturase enzymes. 71. and 72.
Increased understanding of the role of an optimally functioning skin barrier has implications for both the prevention and treatment of atopic disease. While it was previously believed that inhalant allergens were the most common cause of atopy, new evidence suggests that contact allergens are most important. Avoidance of known allergens and frequent bathing to reduce time of exposure and penetration into the skin are now routinely recommended for pets with atopic disease. 73 Because secondary staphylococcal infections may be responsible for many of the clinical signs of atopy, antimicrobial treatment is also an important component of management. Glucocorticoid or cyclosporine therapies are typically prescribed to control inflammation. Fatty acid therapy that helps to restore defects in the intercellular ceramides of the stratum corneum may also be helpful and may allow reduction or discontinuation of the use of these antiinflammatory medications. In recent years, fatty acid therapy for atopic disease has focused both on modulating the inflammatory response by altering the types of eicosanoids produced by the mast cells and other skin cells involved in the inflammatory response (keratinocytes and cutaneous antigen-presenting cells), and providing nutrients that support a healthier skin barrier. 74
Fatty Acid Supplementation
Supplements that are enriched with omega-3 fatty acids are frequently recommended in the management of inflammatory skin disease in dogs and cats. Omega-3 fatty acids included in these supplements are the PUFAs, EPA (20:5n-3), and DHA (22:6n-3), which are found in certain types of fish oil. ALA (18:3n-3), found in flax, has also been used. However, the limited ability of adult animals to convert ALA to long-chain PUFAs for incorporation into cell membranes suggests that this omega-3 fatty acid has less value as a supplement for affecting the production of inflammatory mediators. In addition to omega-3 fatty acids, the omega-6 fatty acid LA is needed for normal epidermal lipid barrier function. Therefore supplementation with LA may result in reduced transepidermal water loss. 33
A second, less common omega-6 fatty acid that has been studied for its antiinflammatory effects is GLA. Once consumed, GLA is readily converted to dihomo-gamma-linolenic acid in the liver, where it is then further metabolized to either the monoenoic prostaglandins and thromboxanes or to AA (Figure 31-5). The more active pathway is toward the production of the monoenoic prostaglandins (PGE 1) because the rate-limiting delta-5-desaturase step for AA production is quite slow in animals. 41 Like the eicosanoids that are produced from EPA, PGE 1 is less inflammatory than the dienoic prostaglandins that are produced from AA. Therefore it is expected that providing supplemental GLA will promote the formation of dihomo-gamma-linolenic acid and the monoenoic prostaglandins, rather than the formation of AA and its more inflammatory metabolites.
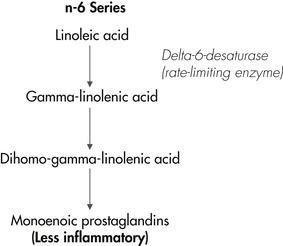 |
| Figure 31-5 |
When dogs’ diets are supplemented with GLA, plasma levels of dihomo-gamma-linolenic acid increase. 75 Similarly, when dogs with atopy that had been controlled by feeding a supplement containing omega-3 fatty acids and GLA were switched to a supplement containing only olive oil (a poor source of both omega-3 and omega-6 fatty acids), plasma levels of dihomo-gamma-linolenic acid subsequently decreased. 76 A return of clinical signs in these dogs paralleled the reduction in dihomo-gamma-linolenic acid. As stated previously, it has been theorized that one factor that may contribute to atopic disease in some dogs is low activity of the enzyme delta-6-desaturase, the rate-limiting step for conversion of LA to GLA. 77 Providing GLA to this subpopulation of dogs would be expected to bypass this step of fatty acid metabolism and provide substrate for the production of the monoenoic prostaglandins. However, not all studies have shown a benefit of providing GLA to pruritic dogs. A study comparing pruritic and healthy dogs found that the unmedicated dogs with signs of atopy naturally had higher concentrations of dihomo-gamma-linolenic acid in subcutaneous fat when compared with levels in the dogs with healthy skin. 78 These results suggest that some dogs with atopy may have an abnormality in dihomo-gamma-linolenic metabolism caused by a deficiency in delta-5-desaturase. Together these studies suggest that there may be several underlying causes and influencing factors that contribute to atopy in dogs. While some atopic animals show abnormal fat absorption and clearance, others may have deficiencies of delta-6- and/or delta-5-desaturase activities, and/or, as examined more recently, impairments that affect the epidermal barrier.
Using supplementation of pets’ regular diets with omega-3 and omega-6 fatty acids to manage the pruritus and inflammatory responses associated with atopy has met with variable success. A review of five separate clinical tests of a commercial supplement (DVM Derm Caps) shows that fatty acid supplementation was effective in controlling pruritus in 11% to 27% of dogs with inflammatory skin disease. 79.80.81.82. and 83. This supplement contains EPA, DHA, LA, and GLA. In one study, 93 dogs with a diagnosis of atopic dermatitis were supplemented at a dose that provided 15 mg EPA per 9 kg (20 pounds [lb]) of BW. 79 One third of the dogs showed a good or excellent response to the supplement, and 17 dogs (18%) required no additional therapy. A second study using the same supplement reported that 11% of dogs with atopy, food allergy, or flea-bite allergy were adequately controlled by the supplement alone with no other treatment necessary. 80 Because these studies did not control dogs’ regular diets, variability of the background fatty acid intake among dogs fed different commercial foods may have significantly influenced response rate. A more recent study fed 22 atopic dogs the same balanced homemade food and administered a daily supplement containing 17 mg/kg BW EPA, 5 mg/kg BW DHA, and 35 mg/kg BW GLA. 84 Supplementation resulted in an overall omega-6 to omega-3 ratio of 5.5:1. The dogs in this study were also divided into two groups: early stage (preimmunotherapy) and late-stage (nonresponsive to immunotherapy). More than half (53%) of the early-stage dogs responded positively to fatty acid supplementation, compared with only one dog from the group of seven dogs that had chronic and refractory atopy. Interestingly, plasma fatty acid profiles also differed between the two categories of atopic dogs, even though they were fed the same diets and supplements. This difference supports the theory that subpopulations of atopic dogs exist, whose disease is influenced by different factors, which in turn could affect clinical response to supplementation.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree