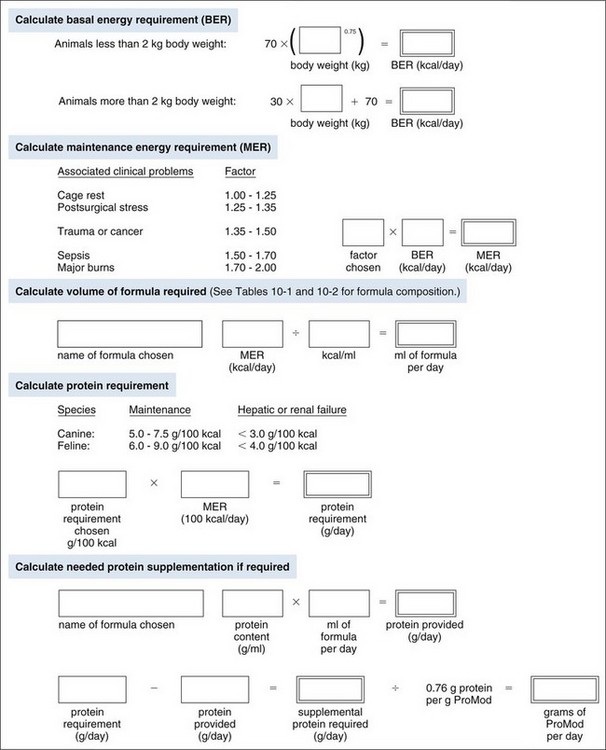
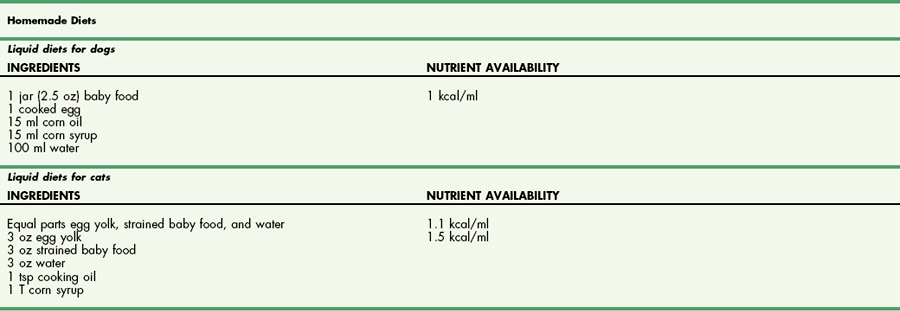
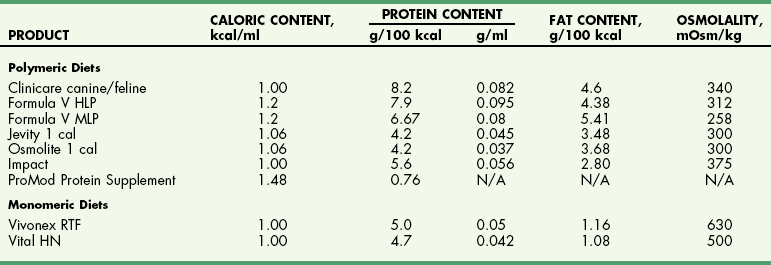
Chapter 10 Care of the surgical patient does not end when the procedure is finished. Postoperative care of surgical patients often determines the ultimate outcome; with critical patients, it may determine whether they survive. Postoperative care involves monitoring vital signs, normalizing homeostasis, controlling pain (see Chapter 12), and recognizing complications early. Early recognition of potentially catastrophic conditions facilitates treatment and ultimately recovery. Recommendations for postoperative care of animals with specific diseases or after particular procedures are included throughout this textbook. Diagnosis of PCM is possible if three or more of the criteria listed in Box 10-1 are present. Physical examination may reveal poor hair coat, pressure sores or wounds that will not heal, tissue wasting, skeletal muscle atrophy, emaciation, or all of these. Additional physical findings vary depending on the cause of malnutrition. Thoracic and abdominal radiographs of malnourished patients generally are nonspecific. Imaging techniques occasionally reveal an underlying cause for the patient’s hyporexia, anorexia, or emaciation, such as an intestinal obstruction or an abdominal or thoracic mass. Biochemical changes with PCM may include hypoproteinemia, anemia, hypoglycemia, hyperglycemia, hyperlipidemia, or a combination of these. Other changes may be related to the specific underlying disease. Specific treatment depends on the patient’s calculated energy needs, the dietary formula chosen, and the route of administration (i.e., enteral, parenteral, or partial parenteral). Basal energy requirement (BER) (also called resting energy requirement [RER]) is based on body weight. Traditionally, maintenance energy requirement (MER) is then determined by multiplying the BER by an arbitrary factor (Fig. 10-1) to accommodate for the presumptive increase in metabolism associated with the severity of the clinical problem (i.e., cage rest, postsurgical stress, trauma, cancer, sepsis, or major burn). Recently, a more conservative approach of starting with BER has been advocated to avoid overfeeding and subsequent complications. Such complications include hyperglycemia, gastrointestinal upset, hepatic dysfunction, and increased carbon dioxide production. The ideal enteral dietary formula should be well tolerated and easily digested and absorbed; it should contain essential nutrients, be readily available and inexpensive, have a long shelf life, and be easy to use. The most cost-effective, well-balanced diets for enteral administration are those blenderized from prescription pet food or homemade diets. Caloric density and protein content vary with the diet chosen. Examples of blenderized diets and their compositions are given in Table 10-1. Blenderized diets can be administered through tubes with a diameter of size 8 French (Fr) or larger; a commercially available liquid diet is recommended for feeding through smaller-diameter tubes (e.g., 5 Fr). Blenderized Diets for Dogs and Cats *Predicted as fed energy density of blended mixture. From Hand M: Small animal clinical nutrition, ed 5, 2010, Mark Morris Associates. Liquid diets should be isotonic (i.e., approximately 300 mOsm/L), have a caloric density of approximately 1 kcal/ml, include fiber at 1 to 1.5 g/100 kcal, and provide approximately 16% of total calories as protein (i.e., protein content of at least 4 g/100 kcal) and approximately 30% as fat. Liquid diets for enteral use generally are categorized as monomeric (elemental) or polymeric. Monomeric diets use small-molecular-weight compounds for nutrients such as crystalline amino acids as the protein source, glucose and oligosaccharides as the carbohydrate source, and medium- or long-chain triglycerides as the essential fatty acid source. These diets generally have twice the normal osmolality and can be used in patients with malabsorptive or inflammatory gastrointestinal disorders (e.g., short bowel syndrome, severe inflammatory bowel disease) but are expensive. A commonly used commercially available monomeric elemental diet is Vivonex RTF (Nestle Healthcare Nutrition, Inc., Florham Park, N.J.; composition shown in Table 10-2). Commercially Available Diets and Their Composition* *These figures should be used for the energy requirement calculations in Figure 10-1. Polymeric enteral diets contain complex large-molecular-weight proteins, carbohydrates, and fats. These diets approach isotonic osmolality, require normal gastrointestinal digestive processes, supply about 1 kcal/ml, and are more economical than monomeric diets. Polymeric diets include blenderized diets, commercially available partially hydrolyzed diets, and commercially available liquid diets (see Tables 10-1 and 10-2). Commercial polymeric diets are available in a variety of osmolalities, caloric densities, and compositions. Examples of commonly used commercial polymeric diets and their compositions are listed in Table 10-2. These diet formulas are indicated for malnourished patients with intact digestive and absorptive function and those suspected of having food allergies. These diets also should be used for patients that must be fed through small-diameter tubes, such as nasoesophageal, gastroduodenostomy, or enterostomy tubes. Polymeric liquid enteral diets have been shown to be effective in providing for nutritional support in critically ill and injured animals. Diets available for TPN should be customized to meet an animal’s protein, carbohydrate, and fat requirements; caloric needs are calculated as described in Figure 10-1. A common composition is 8.5% amino acids with electrolytes (protein source), 10% to 20% lipids (fat source), and 50% dextrose (carbohydrate source). B-complex vitamins are added at 1 to 2 ml/L. In vitro studies have shown that parenteral nutrition formulas do not directly contribute to colloid oncotic pressure; however, TPN has been shown to positively affect nitrogen balance, wound healing, and patient recovery. Possible problems include catheter management (i.e., sterile placement, maintaining sterility of the catheter entrance, and routine changing of infusion sets), expensive equipment (i.e., infusion pump), expensive feeding formulas, technical problems (i.e., routine patient monitoring during administration, proper diet preparation, and storage), metabolic complications, and sepsis. In addition, if the gastrointestinal tract is not adequately stimulated by luminal nutrients and hormonal or neurovascular mechanisms, the intestines and the pancreas may atrophy. Intestinal mucosal compromise predisposes the intestinal mucosa to bacterial translocation into the portal circulation and could possibly lead to sepsis. These problems make parenteral hyperalimentation less desirable than enteral hyperalimentation. Oral feeding is preferable to parenteral nutrition if adequate nutrients can be consumed to meet protein and caloric requirements. Several techniques have been used successfully to encourage an animal to eat. If the owner can manage the patient at home, the likelihood of eating is greater in a familiar environment. Petting and vocal reassurance are also helpful, albeit time-consuming. Highly palatable foods or food coverings, such as gravy, may stimulate the appetite, while warming foods increases aroma and palatability. Supplementing potassium (i.e., 0.5 to 1 mEq/kg per os), vitamin B complex (in maintenance fluids), and/or zinc may also increase appetite. Multiple pharmacologic appetite stimulants are available; recommended dosages are listed in Box 10-2. These drugs are rarely adequate for stimulating a severely anorexic animal to eat sufficiently, but they may stimulate partially anorexic patients to resume eating. The predetermined nutrient formulation (based on calculations described earlier and shown in Figure 10-1) should be administered using an infusion pump. Generally, 50% of the calculated nutrient requirement should be administered the first day and 100% the second day. Initially, serum electrolytes, phosphorus, glucose, albumin, triglycerides, packed cell volume (PCV), and blood urea nitrogen (BUN) concentrations are evaluated daily, and body weight and temperature at least twice daily. In patients receiving TPN for several weeks, biochemical monitoring is often decreased to once every 2 to 3 days, depending on the patient’s condition. Every 2 days, the neck bandage should be removed, the catheter entrance site cleaned with a povidone-iodine or chlorhexidine solution, the extension and administration sets changed, and a new bandage applied. PPN can be administered through a peripheral catheter because partial parenteral solutions are not as hypertonic as TPN solutions. Although a dedicated catheter is ideal, it is not as critical with PPN as it is with TPN. PPN is generally administered on a short-term basis to nondebilitated patients that are unable to tolerate full enteral feeding. Calculations for formulating PPN diets are listed in Box 10-3. The median duration of feeding in dogs and cats receiving PPN is reported to be 3 days (Chan et al, 2002). Patients receiving PPN initially are monitored once a day in the same way as patients receiving TPN. Complications (e.g., sepsis, phlebitis, hyperglycemia, other metabolic derangements) are less common with PPN than with TPN. Generally, oral alimentation is more efficient, easier, and safer and allows greater flexibility in formula composition. The farther aboral materials are delivered, the less efficient are the assimilation and digestion of nutrients, and the greater is the care necessary in choosing formula composition. Route of administration dictates feeding tube diameter (Box 10-4), which in turn dictates the usable feeding formulas. Various formulas have different viscosities and particulate matter size (see previous discussion). The most common routes of administration for enteral hyperalimentation are oral, nasoesophageal, pharyngostomy, esophagostomy, gastrostomy, gastroduodenostomy, and enterostomy routes. Each route has its indications, contraindications, advantages, disadvantages, and complications. Nasoesophageal intubation is easy, effective, and efficient. The availability of small-bore, soft rubber (polyvinyl chloride), and Silastic feeding tubes (i.e., 5 Fr) and of low-viscosity, nutritionally complete liquid diet formulations (see Table 10-2), in addition to patient tolerance of tube placement, have made nasoesophageal feeding popular. Advantages of nasoesophageal tubes include ease of placement, acceptance by patients, ease of tube care and feeding, patients’ ability to eat and drink around the tube, and flexibility that allows tube removal at any time after placement. Major disadvantages of nasoesophageal tubes include the small size of the tube, inadvertent tracheal placement, and premature removal by the patient. Occasionally, a patient may vomit out or regurgitate the tube. Light general anesthesia may be necessary to place a nasoesophageal tube, but a topical anesthetic or light sedation is usually sufficient. Instill proparacaine hydrochloride (0.5 to 1 ml; 0.5%) into the nasal cavity and elevate the head to encourage the anesthetic to coat the nasal mucosa. Repeat the application to ensure adequate anesthesia of nasal mucous membranes. If the patient will not tolerate nasal intubation, consider topical lidocaine (e.g., 1 to 2 ml of 2% lidocaine), heavy sedation, or light general anesthesia. Select an appropriate-size feeding tube (see Box 10-4). Estimate the length of tube to be placed in the esophagus by measuring the tube from the nasal planum, along the patient’s side, to the seventh or eighth intercostal space. Place a tape marker on the tube once the appropriate measurement has been taken. Do not allow the feeding tube to pass through the lower esophageal sphincter because this may result in sphincter incompetence, esophageal reflux of hydrochloric acid, esophagitis, and vomiting. Before passing the tip of the tube, lubricate it with 5% viscous lidocaine and hold the patient’s head in a normal functional position (i.e., avoid hyperflexion or hyperextension). Identify the prominent alar fold and direct the tube from a ventrolateral location in the external nares to a caudoventral and medial direction as it enters the nasal cavity (Fig. 10-2). When the tube has been introduced 2 to 3 cm into the nostril, contact with the median septum at the floor of the nasal cavity can be felt. Push the external nares dorsally to facilitate opening of the ventral meatus. Elevate the proximal end of the tube and advance it into the oropharynx and esophagus. It will generally “drop” into the oropharynx, stimulating a swallowing reflex. Several methods can be used to confirm esophageal placement: (1) check for negative pressure, (2) inject 3 to 5 ml of sterile saline through the tube and see if a cough is elicited, (3) inject 6 to 12 ml of air and auscultate for borborygmus at the xiphoid, (4) connect the tube to a capnograph, or (5) visualize tube placement using a thoracic radiograph. If the patient requires general anesthesia, correct tube placement can be visually confirmed. Once satisfied that the tube has been placed properly, suture it to the nose and head to prevent removal by the patient. In cats, it is important that the tube is not in contact with the whiskers; position it directly over the dorsal aspect of the nose and forehead (Fig. 10-3), and secure it with a Chinese finger-trap friction suture (see Fig. 31-10 on p. 999). In dogs, secure the tube to the lateral aspect of the nose and the dorsal nasal midline with a Chinese finger-trap friction suture or cyanoacrylate glue. Place a column of water in the tube before capping it to prevent air intake, reflux of esophageal contents, or occlusion of the tube by diet.
Nutritional Management of the Surgical Patient
Diets for enteral use
![]() TABLE 10-1
TABLE 10-1
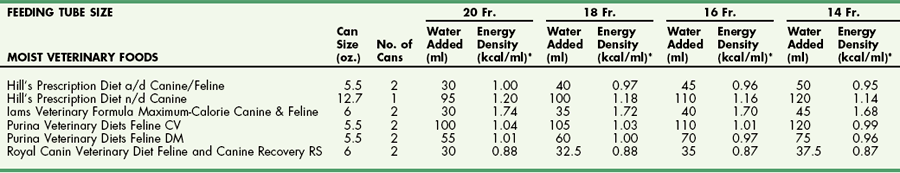
![]() TABLE 10-2
TABLE 10-2
Diets for total parenteral nutrition
Diets for partial parenteral nutrition
Methods of Providing Hyperalimentation
Partial Parenteral Nutrition
Enteral Hyperalimentation
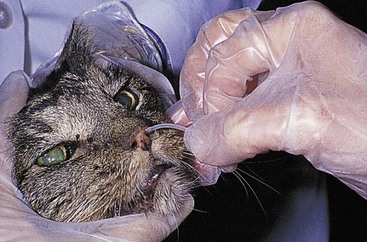
Nasoesophageal Intubation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nutritional Management of the Surgical Patient