Chapter 35. Nutritional Management of Gastrointestinal Disease
Gastrointestinal disease in dogs and cats is composed of a group of disorders with varying and often unrelated underlying causes. Regardless of the cause, most gastrointestinal disorders manifest as acute or chronic diarrhea and, in some cases, vomiting or anorexia. Nutritional support is an important component of treatment because of the gastrointestinal tract’s essential role in nutrient digestion and absorption. A primary goal of dietary therapy is to maintain delivery of nutrients and prevent nutrient deficiencies and malnutrition. In addition, long-term dietary management can help to repair the damaged intestinal lining, restore normal populations of intestinal microflora, promote normal gastrointestinal motility and function, support immune function, and reduce gastrointestinal inflammation. 1. and 2. While dietary management will not always cure the underlying disease, it can have a profound influence on the ability of the intestine to recover and is an important component of veterinary treatment for the control of many types of intestinal disease.
NUTRITIONALLY RESPONSIVE GASTROINTESTINAL DISEASES
Intestinal disorders in companion animals that have been shown to be responsive to dietary management include small intestinal bacterial overgrowth (SIBO)/antibiotic-responsive diarrhea (ARD), pathogen overgrowth, exocrine pancreatic disease, several types of inflammatory disorders, and nonspecific acute diarrhea.
SIBO and ARD
SIBO occurs when there are quantitative and qualitative changes to bacterial populations in the lumen of the proximal (upper) part of the small intestine. 3. and 4. Some researchers have recently argued that SIBO should be renamed antibiotic-responsive diarrhea (ARD) because of the difficulty in SIBO diagnosis via bacterial population numbers, and because not all dogs that are treated successfully for SIBO show reduced intestinal bacterial counts following treatment. 4 The designation of ARD reflects the leading theory that antibiotic therapy and dietary treatments for SIBO support a change in the population dynamics of the intestinal flora, as opposed to simply a reduction in total number of microbes that are present. The goal is to shift the balance of the microbiota away from pathogens and toward the less pathogenic and beneficial species that are present in a healthy intestine. To reflect this change (and the lack of total consensus), the inclusive term SIBO/ARD will be used in this review.
Although some dogs with SIBO/ARD do not have persistent clinical signs, most develop chronic episodes of intermittent diarrhea that may be accompanied by vomiting or anorexia. SIBO/ARD may develop secondarily to a number of other intestinal disorders or be idiopathic. Factors that can lead to secondary SIBO include impaired gut motility, the prolonged or excessive use of oral antibiotics, and achlorhydria (decreased acid production in the stomach). In a healthy animal, bacterial populations increase in number proximally to distally in the gastrointestinal tract. Normal peristalsis helps to limit bacterial populations in the upper small intestine by regularly flushing bacteria distally through the gastrointestinal tract. Reduced motility allows increased substrate to be available to gut bacteria, which can lead to increased microbial populations and changes in the balance of normal flora. Numerous health problems can contribute to a reduction in gut motility and increase risk of developing secondary SIBO. These include intestinal obstruction, neuropathy, abdominal surgery, peritonitis, pancreatitis, uremia, hypokalemia, and endotoxemia. SIBO/ARD may also develop as a sequela to other forms of intestinal disease such as lymphocytic-plasmacytic enteritis and exocrine pancreatic insufficiency (EPI). 5. and 6. Animals with idiopathic SIBO/ARD will have a decrease in signs following the appropriate antibiotic regimen. Finally, SIBO may have a genetic component. German Shepherd Dogs have been reported to have an unusually high incidence of SIBO, and this has been associated with a breed-specific deficiency of secretory immunoglobulin A. 7. and 8.
Historically, the gold standard for diagnosis of SIBO has been via microbiological culture of duodenal fluid obtained endoscopically or during a laparotomy. A culture showing more than 105 total or 104 anaerobic colony-forming units of bacteria per milliliter (CFU/ml) is considered to be consistent with SIBO. 9 However, the clinical relevance of these numbers has been questioned because some healthy dogs consistently have counts that are this high and because a proportion of dogs with ARD/SIBO that respond to antibiotic treatment do not show a reduction in total bacterial counts. 9.10. and 11. For this reason, a total bacterial count in excess of 107 to 108 CFU/ml has been suggested by some researchers as the standard for diagnosis of SIBO in dogs and cats. 10
In addition, elevated serum folate, reduced serum cobalamin, and elevated deconjugated bile acids provide indirect supportive evidence for bacterial overgrowth in the proximal small intestine. 12.13. and 14. Although serum folate and cobalamin are individually not very sensitive indicators of SIBO, when these values are concurrently altered, these changes are considered specific for SIBO/ARD. Serum folate increases as bacteria numbers in the upper small intestine increase due to increased bacterial production of folate. Serum cobalamin concentration decreases because bacteria interfere with cobalamin binding to intrinsic factor and subsequently inhibit absorption of the vitamin into circulation. Intestinal bacteria also cause the deconjugation of bile salts, which are then reabsorbed, making them unavailable to participate in fat absorption. Deconjugated bile acids that remain in the intestinal lumen are also a significant contributor to the osmotic and secretory diarrhea seen in dogs with SIBO/ARD.
Qualitative changes in the bacterial flora of the small intestine of pets with SIBO/ARD are as significant as increased numbers, and these changes should always be assessed. Species of bacteria that typically increase in dogs with SIBO/ARD include coliforms, staphylococci, and enterococci, with Clostridium and Bacteroides species predominating. Although clinical SIBO is less common in cats, subclinical cases have been reported based upon earlier diagnostic criteria. 15 The most common species of bacteria found in cats with SIBO were Bacteroides species, eubacteria, fusobacteria, and Pasteurella species. In addition, the cat appears to be unique in the relatively high number of clostridia found in the intestine, as compared with other carnivorous species. Even when the challenges of defining and diagnosing SIBO/ARD are considered, it is still generally accepted that SIBO/ARD is an important cause of chronic diarrhea in dogs (and less commonly in cats), as well as a concomitant finding in several other forms of chronic intestinal disease. 16
SIBO/ARD occurs when there are quantitative and qualitative changes to bacterial populations in the lumen of the proximal (upper) part of the small intestine. Clinical signs include chronic episodes of intermittent diarrhea that may be accompanied by vomiting or anorexia. SIBO/ARD may be a primary disease or can develop secondarily to a number of other intestinal disorders.
Pathogen Overgrowth
In healthy animals, the many genera and species of intestinal microbes exist in a commensal balance that promotes normal digestive and immune functioning. 17.18. and 19. When present in optimal numbers, bacterial species that are classified as beneficial inhibit the proliferation of harmful bacterial species, stimulate immune function, aid in the digestion or absorption of food, and synthesize essential vitamins. 20 These effects are achieved through competition for oxygen, luminal substrates, and living space within intestinal niches. In addition, some indigenous flora produce substances that directly inhibit the growth of other bacterial species. For example, saccharolytic species of intestinal microbes produce short-chain fatty acids (SCFAs) during the metabolism of carbohydrate. In turn, these SCFAs inhibit the growth of some pathogenic species of bacteria. 21
An animal’s intestinal microbial population is not static. Microbial populations are susceptible to change and can be affected by stress, infection, antibiotic administration, nutritional factors, motility problems, and immunosuppression. When a change in the normal population of microbes allows the proliferation of one or more pathogenic species of bacteria, clinical illness can result. Pathogen overgrowth may manifest as a single problem or can occur secondarily as a component of SIBO/ARD or another form of intestinal disease. The proliferation of pathogenic species of bacteria causes harm to the host animal by producing toxins, carcinogens, or putrefactive compounds. These compounds may directly affect the intestinal mucosa, cause systemic disease, and inhibit the growth of beneficial bacteria. One of the most common intestinal pathogens in companion animals is Clostridium perfringens. 19. and 22. Signs of an imbalanced intestinal microbe population or pathogen overgrowth include vomiting, diarrhea, weight loss, and, in some cases, systemic illness caused by the production of toxins.
Pancreatitis and Exocrine Pancreatic Insufficiency
Pancreatitis and EPI are well-defined gastrointestinal disorders in dogs and cats that can occur in acute and chronic forms. 23. and 24. Acute pancreatitis is a short-term, usually reversible illness in which there is no evidence of tissue fibrosis or acinar atrophy. 25 It may have an extremely rapid onset, and in severe cases may lead to rapid tissue necrosis and a potentially lethal outcome. Chronic pancreatitis is a continuous, usually progressive inflammatory disorder that is characterized by permanent damage to pancreatic structure and exocrine (and sometimes endocrine) function. Acute pancreatitis is the most common form that is diagnosed in dogs, while chronic pancreatitis is the primary manifestation seen in cats. 26 However, dogs treated for acute pancreatitis may subsequently develop chronic or subclinical pancreatitis. There is also recent evidence suggesting that many cases of chronic pancreatitis in dogs are undiagnosed. 27 Similarly, because feline pancreatitis may be subclinical or mild, cases of chronic feline pancreatitis may be easily missed. 28
The underlying cause of many cases of pancreatitis is not determined, so many cases are classified as idiopathic. Identified risk factors for developing pancreatitis include being overweight or obese, hyperlipidemia, consuming a high-fat meal or experiencing one or more bouts of dietary indiscretion (dogs), and the presence of other forms of gastrointestinal disease such as inflammatory bowel disease or liver disease (cats), neoplastic disease, and exposure to certain drugs or infectious agents. 25.29.30. and 31. Cats are susceptible to a generalized inflammatory syndrome called “triaditis,” in which chronic pancreatitis occurs concurrently with inflammatory diseases of the liver and intestine. 31 In dogs, Miniature Schnauzers and Yorkshire Terriers may be at increased risk for developing pancreatitis, and a possible mode of inheritance has been identified in Miniature Schnauzers. 29. and 32.
EPI occurs when the exocrine pancreas produces inadequate amounts of digestive enzymes resulting in a maldigestion/malabsorption syndrome. In dogs, the most common underlying cause of EPI is selective atrophy of the pancreatic acinar cells, which are responsible for the production and storage of the digestive enzymes. 33 Canine EPI has a genetic basis; the majority of cases are diagnosed in German Shepherd Dogs and Rough Collies. 34 The mode of inheritance in these two breeds appears to be autosomal recessive. A recent report also found that Cavalier King Charles Spaniels and Chow Chows are also at increased risk for EPI when compared with the entire population of dogs, and there appears to be a juvenile-onset form of EPI in Greyhounds. 35. and 36. EPI is less common in cats, but can occur as a sequela to chronic pancreatitis. A genetic basis for EPI has not been reported in cats.
Although acute pancreatitis can be a medical emergency, most cases of chronic pancreatitis are classified as mild, with the pet showing transient but recurrent signs. The most common clinical signs of pancreatitis are complete or partial anorexia, depression and lethargy, vomiting, and weight loss. 24 Pets often present with abdominal pain, dehydration, icterus, and dyspnea. The clinical signs of EPI all relate to the reduced ability to digest and absorb dietary nutrients. Fat digestion and absorption is severely impaired, which can contribute to both acute and chronic intestinal symptoms. 37 Other changes in intestinal function include a decrease in protein synthesis within enterocytes, malabsorption of some vitamins, and secondary development of SIBO/ARD or pathogen overgrowth. Studies indicate that SIBO/ARD is found in more than 70% of dogs with a diagnosis of EPI. 38 Animals with EPI produce voluminous stools that are often loose and foul smelling. Frequent bouts of diarrhea and steatorrhea are common. Excessive weight loss and an emaciated appearance are classic signs of EPI, even while the pet demonstrates a voracious appetite and polyphagia. The diarrhea caused by EPI is typically osmotic due to the passage of malabsorbed diet components along the intestinal tract. However, secretory diarrhea of the lower small intestine and colon may also be present, as a result of bacterial deconjugation of bile acids and the metabolism of unabsorbed fat to hydroxy fatty acids.
While clinical signs and history are helpful in diagnosing pancreatitis and EPI, definitive diagnosis is made using results of the pancreatic lipase immunoreactivity assay (pancreatitis) or the serum trypsin–like immunoreactivity assay (EPI). 39.40.41. and 42. Immediate treatment of acute pancreatitis requires fluid therapy and correction of hypokalemia or other electrolyte or acid-base imbalances, if present. 25 Pain management is also important for pets with pancreatitis and is typically achieved via opioid administration or transdermal fentanyl patches. There is evidence that pancreatic enzyme supplementation is helpful at reducing pain in human patients with chronic pancreatitis. Although it has not been studied, this may be helpful in dogs and cats as well. Early nutritional intervention via enteral feeding also may be beneficial in acute cases of pancreatitis. An important component to the long-term management of both pancreatitis and EPI is diet modification. For pets with pancreatitis, reduction of overweight conditions (if present) is important, and possibly the control of dietary fat (see pp. 463-464). Long-term management of EPI includes providing supplemental enzyme extracts with meals and dietary modification to promote efficient digestion and reduce malabsorption. In addition, antibiotic therapy or dietary approaches to control SIBO are necessary for some animals.
Exocrine pancreatic insufficiency (EPI) is a specific type of pancreatic disease that occurs when the exocrine pancreas produces inadequate amounts of digestive enzymes. Clinical signs all relate to the reduced ability to digest and absorb dietary nutrients. Animals with EPI produce voluminous stools that are often loose and foul smelling, and diarrhea and steatorrhea are common. Excessive weight loss and an emaciated appearance are seen, even while the pet demonstrates a voracious appetite. Diagnosis of EPI is made using results of the serum trypsin–like immunoreactivity assay.
Inflammatory Bowel Disorders
Inflammatory diseases of the intestine are a diverse group of disorders that are generally categorized according to the type of inflammatory cell that predominates in the intestinal mucosa, the area of the intestine affected, or the underlying cause, if known. Colitis is a general term for a condition that describes irritation or inflammation of the large intestine, and it is considered the most frequently diagnosed disorder of the large intestine in dogs and cats. 43 Colitis is further classified into several forms, which include lymphocytic-plasmacytic, eosinophilic, histocytic, and granulomatous colitis. Lymphocytic-plasmacytic enterocolitis is the most common form of inflammatory bowel disorder (IBD) in dogs. 44 In recent years, a new classification has identified a subgroup of dogs with chronic idiopathic large bowel diarrhea (CILBD). 45 Although limited data are available, CILBD is presumed to be a stress-related disorder that may be concomitantly influenced by other factors such as inflammatory disease, dietary indiscretions, pathogen overgrowth, parasitic infection, and neoplasia. Clinical signs of colitis and CILBD are the result of dysfunction of the large intestine and include increased defecation frequency, tenesmus, and production of bloody or mucoid diarrhea. When IBD affects the small intestine, signs include production of large quantities of soft, bulky stools or diarrhea, with occasional steatorrhea. Contrary to large intestinal disease, weight loss and vomiting are common with small intestinal IBD.
IBD is considered to be a multifactorial disorder. Predisposing factors include genetics, exposure to luminal antigens and loss of immune tolerance, compromised colonocyte health, increased mucosal permeability, and impairment or insufficiency of the intestine’s normal protective mechanisms. 46 A genetic basis may be involved in some forms of IBD; Boxers and German Shepherd Dogs are at increased risk of developing lymphocytic-plasmacytic enterocolitis while Basenjis are susceptible to a form called immunoproliferative enteropathy. 47.48. and 49. Several forms of IBD occur in the domestic cat, including lymphocytic-plasmacytic enteritis and eosinophilic enteritis, but no breed-specific predilections have been reported. 50. and 51. Additionally, distinguishing between IBD and gastrointestinal lymphoma can be diagnostically challenging in cats. Infection with an intestinal parasite or bacterial pathogen, bacterial overgrowth, or presence of a food antigen may all trigger an initial inflammatory response, which can persist even after the initial cause has been resolved. Once an immune response has been initiated in the small or large intestine, production of inflammatory eicosanoids and tissue ischemia may sustain the inflammatory response, resulting in a cycle of chronic illness.
Treatment of IBD and CILBD is directed toward eliminating the underlying cause (if one can be found), reducing inflammation, and achieving long-term remission. Medical therapy of IBD includes the use of antiinflammatory and immunosuppressive drugs such as corticosteroids (prednisone or prednisolone), sulfasalazine, and, in some cases, oral cyclosporine or metronidazole (cats). 52 However, these drugs are associated with undesirable and deleterious health effects when used for long periods. In recent years, new regimens have been developed that focus on dietary management (see pp. 461-472 for complete discussion). 52. and 53. While drug therapy is still often used in the initial treatment phase to reduce inflammation and allow healing of the intestinal tract, dietary management can often maintain remission and prevent relapse. Drug therapy is then only reinstated if clinical signs return.
Nonspecific Acute Diarrhea
Nonspecific acute diarrhea refers to short episodes of diarrhea for which a cause cannot be found. In most cases, the pet remains active, there is no evidence of systemic disease, and the clinical signs are self-limiting. In dogs, this condition is commonly caused by dietary indiscretions such as consuming garbage, animal feces, or carrion. Overeating or a sudden change in the type or brand of food that is fed can cause acute diarrhea in both dogs and cats. Feeding a poorly formulated or inadequately prepared homemade diet or excessive amounts of table scraps can also lead to small or large intestinal diarrhea. In these cases, the diarrhea can be treated symptomatically until the animal recovers or an underlying cause can be found and treated.
Gastric Dilatation-Volvulus
Gastric dilatation-volvulus (GDV), commonly referred to as bloat, is a life-threatening disorder characterized by rapid and abnormal distention of the stomach (dilatation). This disorder is often, but not always, accompanied by rotation of the stomach along its long axis (volvulus). Dilatation occurs when gas, fluid, and secretions accumulate within the stomach and are not expelled because of the occlusion of both the cardiac and pyloric sphincters. The condition rapidly worsens as the distended and rotated stomach places pressure on the major abdominal blood vessels, the portal vein, and caudal vena cava. This pressure causes a loss of blood flow to the stomach and other vital organs, decreased cardiac output, and the development of shock. At this point the dog’s condition rapidly deteriorates, and if the GDV is not corrected quickly, shock and tissue damage become severe and death ensues. In addition, cardiac arrhythmias are observed in up to 40% of dogs with GDV and can cause death within weeks or months following apparent recovery from GDV. 54 Diagnosis is usually based on clinical signs, with radiographs used to distinguish between GDV and gastric dilatation without gastric torsion. Although the course of GDV can be quite variable among dogs, this disorder should always be treated as a medical emergency.
Bloat most often affects large, deep-chested dogs. The breeds that have been identified as being most susceptible include the Great Dane, Saint Bernard, Weimaraner, Irish Setter, Gordon Setter, Standard Poodle, and Basset Hound. 55 Although rare, the disorder can also occur in small breeds of dogs and cats. 56 Risk of GDV also increases as dogs age. A dog that is developing GDV will exhibit acute abdominal pain and distention, and it will often whine, pace, salivate, and appear anxious. The dog may attempt to vomit but will be unable to regurgitate any stomach contents. As the problem progresses, hypovolemic shock occurs, characterized by pale mucous membranes, a rapid and weak pulse, increased heart rate, and weakness. In all cases, veterinary care must be provided immediately.
Initial treatment of GDV involves decompression of the stomach and treatment for shock. Surgical intervention is usually also necessary and involves derotation and repositioning of the stomach, followed by prophylactic measures that help to prevent recurrence. Surgery also allows assessment of the damage to the stomach and other organs. Even with treatment, prognosis is often guarded. In a retrospective study of almost 2000 cases, the case fatality rate was 33.6% in dogs treated for GDV at veterinary teaching hospitals. 55 The strongest prognostic indicator appears to be the extent of gastric necrosis that has occurred. Death occurs either during surgery because of irreversible shock or within several days of surgery as a result of cardiac complications or gastric necrosis. 57 While nonsurgical treatment is associated with a high rate of recurrence, the use of gastropexy to surgically stabilize the stomach significantly reduces recurrence and postoperative mortality. 58
GDV is considered to have a multifactorial etiology. Potential influencing factors that have been studied include genetic predisposition, dietary management practices, diet type and composition, and intrinsic abnormalities such as elevated serum gastrin or altered gastric motility. Genetics plays an important role in GDV to the degree that body type and structure are inherited characteristics. A study reported that the two most important risk factors affecting a breed’s predisposition for development of GDV are a relatively high chest depth-to-width ratio and a large adult size. 59 Together, chest conformation and body size account for 76% of the variability in breed risk for GDV. Subsequent studies with Irish Setters have shown that there is a positive and significant correlation between increasing chest depth-to-width ratio and an individual dog’s risk for developing GDV. 60 Because chest depth-to-width ratios are significantly influenced by genetics, it may be possible to reduce the incidence of GDV through selective breeding. 61 Studies of the genetics involved in GDV are complicated by the fact that it is often difficult or impossible to separate these effects from environmental influences such as husbandry practices, medical care, and feeding management. However, dogs that inherit a large, deep-chested body type have an increased susceptibility to this disease, and this conformation appears to have a high heritability in some breeds of dogs.
Other studies have identified additional risk factors for GDV. 62. and 63. While some of these, such as breed and conformation, are characteristics that are inherent to a particular dog, others are environmental factors over which owners have at least some measure of control. In one study, a group of 101 dogs that had acute episodes of GDV were individually matched with dogs of the same age, breed, and size. 63 Comparisons of the two groups showed that physical characteristics that significantly increased an individual dog’s risk of GDV included gender, body weight, and temperament. Specifically, male dogs that were underweight and were determined to have a nervous or fearful temperament were at higher risk for eventually developing GDV. In contrast, a study of 74 Irish Setters with GDV and a group of matched controls found that the risk of GDV was not associated with gender or temperament in that breed. The differences between these two studies may reflect differences between the general population of dogs and the subpopulation of a specific breed (Irish Setters). In both studies, however, the most significant precipitating factor identified was an episode of environmental change or a stress-inducing event within several hours preceding the onset of clinical signs. In the Irish Setters that were studied, two precipitating risks that were identified were recent kenneling and a recent car journey.
Several nutritional factors have been found to influence a dog’s risk for GDV. 62. and 64. These include consuming only one meal per day, having a fast rate of eating, and experiencing aerophagia while eating. On the other hand, feeding table foods, having snacks available between meals, and adding canned food to a dog’s diet all decreased the risk of GDV. This second group of practices all contribute to a more frequent meal schedule and are assumed to result in a decreased volume of food being fed during a single meal. These findings are in agreement with earlier reports that the majority of cases of GDV occur when the dog has recently consumed a large meal or quantity of water. 64 Finally, contrary to popular belief, using an elevated food bowl to feed dogs who are increased risk of GDV does not help to prevent bloat and may even increase risk. 65
The mechanism through which meal frequency affects gastric health may have to do with the volume of food that is fed. A study with Irish Setters reported that dogs fed one large meal per day throughout growth developed larger, heavier stomachs than did dogs fed three meals per day during the same period. 64 Dogs that were fed once daily also had greater gastric distention than did dogs fed multiple meals, but no differences in gastric motility were seen between the two groups. The investigators concluded that feeding one time per day, rather than feeding multiple small meals per day, may contribute to changes associated with GDV in susceptible dogs. It has also been postulated that strenuous exercise, stress, or excitement may be contributing factors, especially before or after a meal or a large volume of water is consumed.
Composition of the diet or type of diet that is fed has received attention as a possible cause of GDV, but there are little actual data to support this theory. Dry dog foods were initially implicated because of the belief that they absorb water and expand while in the stomach, causing an abnormal amount of gastric distention. Another theory proposed that cereal-based, dry diets delay gastric emptying when consumed and contribute to the accumulation of gas in the stomach. The presence of soybean products in pet foods has also been proposed as a causative factor. It has been theorized that soy provides a fermentative substrate for Clostridium spp. bacteria within the stomach, which produces the gas responsible for GDV. However, a study with large breeds of dogs compared the effects of feeding a dry cereal-based diet, canned meat or canned, cereal-based diet on gastric motility and the rate of gastric emptying. Results showed no significant effect of diet on gastric function in any of the dogs that were studied. 66 Another clinical study involving 240 dogs that had been treated for GDV did not find any correlation between the type of food that was fed and the occurrence of GDV. 67 Lastly, although there is some disagreement, the fermentation theory has been largely refuted by the observation that the gas found in the stomachs of dogs with GDV is made up primarily of atmospheric gas, indicating that swallowed air is usually the source of the gas, not fermented stomach contents. 68. and 69. Production of fermentative gas in the stomach of dogs with GDV can occur after death and may lead to the erroneous conclusion that this gas was the initial cause of the disorder. For example, studies of postmortem tissue decomposition have been unable to demonstrate that the presence of Clostridium bacteria in the stomach is primary to the disease, rather than secondary. Currently, the studies that are available support the conclusion that GDV is not a dietary disorder per se, and that its development is not related to any component in pet foods nor to the type of food that is fed.
Although the type of diet and components within the diet are not causal factors in GDV, several feeding management practices can be used to help prevent GDV in dogs that are susceptible or have a history of GDV. In other words, although what the dog eats does not appear to affect the occurrence of GDV, how the dog is fed and the feeding environment can be managed to minimize the chances of GDV. Portion-controlled meal feeding should be used. Several small meals should be fed per day, as opposed to one large meal, to prevent overfilling of the stomach. 65 Similarly, although fresh water should be available at all times, dogs should not be allowed to drink a large volume of water before or after eating, or after exercise. Because dogs often increase their rate of eating or the amount that they eat when in the presence of other dogs, all susceptible dogs should be fed separately, and any stress that may be associated with the feeding environment should be minimized. If possible, feeding times should be scheduled so that the dog is supervised and can be observed for 1 to 2 hours after meals. Lastly, although exercise as a predisposing factor has not been confirmed, it is prudent to withhold exercise for 1 hour before and at least 3 hours after feeding. All dogs that have a susceptible body type or a history of GDV should be carefully monitored for signs of GDV. If signs are seen, veterinary care should be sought immediately (Box 35-1).
BOX 35-1
Use portion-controlled meal feeding as the feeding regimen.
Feed several small meals per day to prevent overfilling of the stomach.
Do not allow the consumption of a large volume of water immediately before or after eating or exercise.
Feed susceptible dogs separately from other animals. If possible, supervise mealtimes.
Do not provide exercise for 1 hour before and 3 hours after meals.
Minimize stress and environmental changes.
If signs of GDV are observed, seek veterinary assistance immediately.
DIETARY MANAGEMENT OF INTESTINAL DISEASE
A diet used to manage gastrointestinal disease should be selected in accordance with the specific disease being treated, the area of the gastrointestinal tract affected, and the ability of the diet to promote healing and maintain remission. Specific diet characteristics that should be considered include protein and carbohydrate sources, level and type of fat, level and type of dietary fiber, potential benefits of prebiotics or probiotics, and diet digestibility.
Managing Acute Disease
The immediate nutritional management of acute gastrointestinal disease usually involves short-term fasting or severely reduced food intake with the intent of providing “gut rest.” This approach is based upon the theory that slowing gastrointestinal tract function by withholding food allows normal gastric and peristaltic contractions to subside and promotes healing of the intestinal lining. 1 Traditionally, after a fasting period of 12 to 48 hours, a bland diet consisting of highly digestible ingredients has been recommended. However, while “resting the gut” may have benefits, especially when the animal is vomiting or diarrhea is severe, the use of “bland diet” has been questioned due to a lack of evidence for its efficacy. 2 Moreover, while use of the term “bland” to describe the refeeding diet enjoys ubiquitous usage, the definition of this term and the specific diet characteristics are vague and poorly defined. While a short-term fasting period to allow gut rest is helpful in the initial treatment stage of some intestinal disorders and may help to prevent hypersensitivities when altered mucosal permeability is present, studies of intestinal disease in dogs and cats show that the type of diet is very important and that diet characteristics other than “blandness” must be considered (see pp. 462-472). In the face of new information supporting the use of specifically formulated diets for the treatment and management of gastrointestinal disorders, it is advised that the term “blandness” be discontinued altogether when attempting to describe a therapeutic diet.
Long-Term Management—Diet Composition
PROTEIN SOURCE
Diseases that affect the small intestine, such as EPI, SIBO/ARD, and some forms of inflammatory bowel disease, can impair protein digestion and absorption. Prolonged malabsorption of dietary protein can lead to protein malnutrition, which further exacerbates existing intestinal disease through reduced mucosal cell protein synthesis and impaired local immune function. 70 Most seriously, a syndrome called protein-losing enteropathy (PLE) occurs when there is rapid and severe loss of protein from the small intestine. 71 Although PLE is most commonly associated with idiopathic lymphangiectasia, it may also represent the end stage of several chronic intestinal disorders. Therefore the diet should provide a high-quality protein source that is easily digested and assimilated and contains all of the essential amino acids in their correct proportions to minimize the risk of protein malnutrition.
Any dietary protein that is not completely digested and assimilated in the small intestine travels to the large intestine, where it can be metabolized by gut microbes, causing changes in the intestinal microflora. 72 A healthy and balanced intestinal microflora is comprised predominantly of saccharolytic (carbohydrate fermenting) bacterial species, most of which are considered to be beneficial gut microbes. 73 Conversely, other species, such as Bacteroides spp. and Clostridia spp. are capable of fermenting polypeptides and amino acids. These organisms are normally present in low numbers; however, when partially digested protein is delivered to the large intestine it provides increased substrate for these pathogenic species, leading to their proliferation and to the production of ammonia, phenols, indoles, and gas. This can lead to large intestinal diarrhea, further exacerbating intestinal disease.
Dietary protein is also important because of its effect on immune-mediated responses in the gastrointestinal tract. In at least some animals, the development of colitis appears to be an immune-mediated response to food antigens that gain access to the colonic lamina propria and submucosa. 72 Once an immune response is triggered, the continuous exposure of the local immune system of the large intestine to an offending antigen results in persistent inflammation and disruption of intestinal function. Some dogs and cats with chronic gastrointestinal disease are also found to have an adverse food reaction or dietary sensitivity and respond positively to a pet food that contains a single and novel protein source (for a complete discussion of adverse food reactions see Chapter 31, pp. 396-402). 74
For all of these reasons, the proteins that are included in diets for dogs and cats with intestinal disease must be highly digestible. When adverse food reaction is suspected, a single-source protein should be fed, preferably one to which the dog or cat has not previously been exposed. Highly digestible protein sources have reduced antigenicity because less intact dietary protein is absorbed into the mucosa of the small intestine and less arrives intact or partially digested in the large intestine. Providing a single protein source also minimizes the chance of feeding a protein to which the pet has been previously sensitized. Because of a possible connection between food antigens and colitis, some authors recommend feeding an elimination diet to treat colitis and other forms of inflammatory intestinal disease. Types of elimination diets and their use in the diagnosis and management of dietary hypersensitivity are described in detail in Chapter 31 (see pp. 399-402).
Several studies of dogs and cats with colitis have shown positive results when the animals are fed an elimination diet containing a single, novel protein source. 75. and 76. Although response rates vary among studies, between 30% and 85% of dogs with idiopathic colitis respond favorably to this type of regimen. Differences in response rates may reflect variations in the diets used with respect to protein source and digestibility. Animals that did not respond with complete remission to a novel protein source often showed some degree of improvement and required lower levels of antiinflammatory medications to achieve and maintain remission. 75 A number of suitable commercially prepared veterinary diets are available. Most are formulated to provide complete and balanced nutrition and include single-source, highly digestible protein (limited-antigen foods).
It has been suggested that animals with IBD are at increased risk of immunological sensitization to food proteins during the initial phase of treatment. 72 Chronic inflammation of the intestinal mucosa can lead to impaired protein digestion and damage to the intestinal lining. As a result, intact food proteins may have a greater chance of gaining access to the lamina propria and stimulating an immune response during periods of active disease. 43 Therefore the novel protein that is fed during the initial phase of therapy may have only short-term benefit. This theory has led to the concept of using an initial “sacrificial protein source” for the first 4 to 6 weeks of diet therapy. The protein source is then changed again to a second novel and highly available source to use as the pet’s maintenance diet. The intent of this procedure is to introduce the second protein only after mucosal inflammation and permeability has decreased, thus minimizing the risk of the second protein resulting in hypersensitivity. Although this approach has theoretical merit, there is currently no clinical or experimental evidence of its efficacy. Because using a sacrifice diet and protein source requires identifying two novel protein sources and changing the pet’s food twice during treatment, most veterinarians and nutritionists are not currently recommending this treatment approach. 77
Proteins that are included in diets for dogs and cats with intestinal disease must be of high quality and highly digestible. When adverse food reaction is suspected, a single-source protein should be fed, preferably one to which the dog or cat has not previously been exposed. Because of a possible connection between food antigens and colitis, it may be helpful to feed an elimination diet to treat colitis and other forms of inflammatory intestinal disease.
CARBOHYDRATE
Similar to protein, a single carbohydrate source that can be easily digested and assimilated should be included in foods formulated for pets with gastrointestinal disease. Because gluten-induced enteropathy is the cause of intestinal disease in some dogs, particularly Irish Setters, it has been suggested to include only gluten-free carbohydrates in diets formulated for intestinal disease. 78. and 79. Cooked and blended white rice is highly digestible and is gluten free. 80 Other gluten-free carbohydrate sources include potato, tapioca, and corn. Potato and tapioca starches are less digestible than rice, while corn may be contraindicated in dogs that have a hypersensitivity to this ingredient. 81 In contrast, wheat, oats, and barley all contain gluten.
LEVEL AND TYPE OF FAT
A reduced fat diet is often beneficial for dogs and cats with gastrointestinal disease. High-fat intake is specifically contraindicated in animals with EPI, postacute pancreatitis, and lymphangiectasia because these diseases all involve severe impairment of fat digestion and assimilation. A low-fat diet is also indicated whenever there is SIBO or reduced surface area in the small intestine. Malabsorption of dietary fat allows bacterial metabolism of unabsorbed dietary fat to hydroxy fatty acids, while bacterial overgrowth contributes to deconjugation of bile salts, both of which stimulate secretory diarrhea in the distal small intestine and colon. 13 A general recommendation is to select a food that contains approximately 11% or 15% or less total fat (on a dry-matter basis [DMB]) for dogs and cats, respectively, with gastrointestinal disease. 82
The well documented antiinflammatory benefits of omega-3 fatty acids suggest that there may be a role for this class of fatty acids in the management of inflammatory intestinal disease. In addition to demonstrated benefits for pets with inflammatory skin disease (see Chapter 31, pp. 386-394), there is also evidence that altering the omega-6 to omega-3 fatty acid ratio to favor production of omega-3 metabolites alters eicosanoid profiles in the intestinal mucosa (Figure 35-1 and Figure 35-2). 82 When dogs were fed diets containing omega fatty acid ratios of 10:1 and 5:1, intestinal and colonic mucosa eicosapentaenoic acid (EPA) (20:5n-3) and docosapentaenoic acid (22:6n-3) concentrations increased and arachidonic acid levels decreased over an 8-week period. Regional differences were seen, with small-intestinal mucosa having a greater concentration of stearic acid (18:0) and linoleic acid (18:2n-6) than colonic mucosa, and colonic mucosa having greater concentrations of eicosatrienoic (20:3n-3) and arachidonic (20:4n-6) acids.
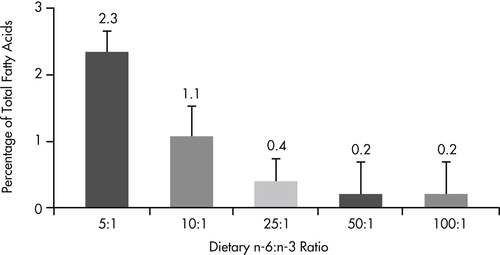 |
| Figure 35-1 (From World Small Animal Veterinary Association: Gastrointestinal health symposium, Dayton, Ohio, 1997, The Iams Company.) |
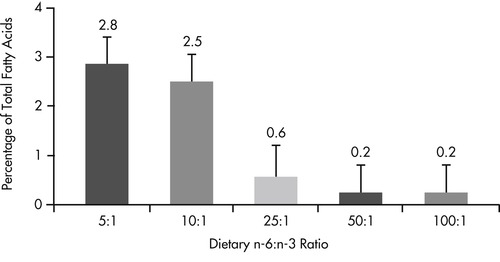 |
| Figure 35-2 (From World Small Animal Veterinary Association: Gastrointestinal health symposium, Dayton, Ohio, 1997, The Iams Company.) < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|