CHAPTER 14 Nutritional Management of Chronic Kidney Disease
Chronic kidney disease (CKD) has been reported to affect one in three cats over the age of 12 years.1 A study by Jepson et al reported that within 12 months, one in three cats aged 9 years or older developed biochemical evidence of azotemia.2 Although CKD is a leading cause of death in cats, progression to an end stage is not inevitable in all cases. Indeed, recent research continues to elucidate key risk factors for progression, and to identify therapies designed to slow disease progression.
Dietary therapy has remained at the forefront of the management of CKD for decades. There are two fundamental approaches of nutrition in CKD: the first is the essential role that certain nutrients have in altering disease progression, and the second is the role of nutrition in controlling clinical signs of uremia and improving the quality of life. Nutritional therapy introduced in Stages II and III of CKD is aimed at factors that delay progression; whereas once late stage III/IV has been reached, clinical signs of the uremic syndrome are evident and dietary treatment is designed more to improve the quality of life of the patient than to slow disease progression (see Chapter 47). Regular monitoring to ensure that dietary and medical management remains optimal for the needs of the patient is crucial for the long-term successful treatment of the patient with CKD.
ENERGY
Cats with CKD often are anorexic or have reduced appetites. However, the efficiency of nutritional therapy depends on the diet being fed consistently and exclusively. Therefore the diet must be palatable enough to avoid any risk of refusal. Practical measures to improve intake include the use of highly odorous foods, warming the foods prior to feeding, and stimulating eating by positive reinforcement with petting and stroking behavior. Appetite stimulants such as the benzodiazepam derivatives or serotonin antagonists (Table 14-1) may be administered judiciously to cats who are hyporexic or show decreased interest in food; however, in many of these cases, more aggressive therapy such as esophagostomy or gastrostomy tube feeding is clinically indicated (see Chapter 12).
Table 14-1 Therapeutic Agents That Can Be Used to Stimulate the Appetite
| Agent* | Conventional Dose | Contraindications |
|---|---|---|
| Cyproheptadine† | 2-4 mg/cat PO q12h | Antiserotoninergic; can cause sedation or paradoxical excitability, aggression and vomiting |
| Oxazepam | 2 mg/cat PO q12h | Contraindicated in hepatic disease |
| Diazepam† | 0.2 mg/kg IV | |
| Mianserin chlorohydrate | 2-4 mg/kg PO q24h | Excitability, aggression, vomiting |
| Mirtazapine | 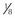 to to 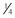 of a 15-mg tablet PO q72h of a 15-mg tablet PO q72h | Excitability, aggression, vomiting |
* Most of these drugs have not been approved for use in the cat.
† Agent undergoes renal excretion and the dosage must be adjusted accordingly to prevent toxicity.
PROTEIN
PROTEINURIA
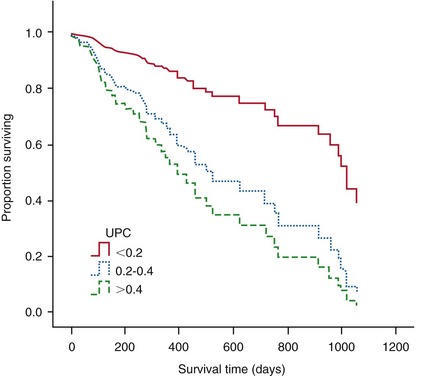
Studies by Syme et al suggested that the upper limit of the reference range for the urine protein : creatinine ratio (UPC) for healthy aged cats is 0.4.3 Recent evidence suggests that the UPC is an independent risk factor for all-cause mortality of cats with CKD,3,4 with systemic hypertension,5 and with uremic crisis6 (see Chapter 49). Plasma creatinine concentration and blood pressure are risk factors associated with proteinuria. Age, plasma creatinine, and proteinuria are significant and independent risk factors associated with reduced survival time of cats with CKD.3 Indeed cats with a UPC greater than 0.4 have a four times higher risk of death compared with cats with a UPC less than 0.2 (Figure 14-1).
Glomerular capillary hypertension, hyperfiltration, and mild proteinuria have been reported in cats following renal mass reduction.7 This leakage of protein into the glomerular filtrate has been implicated in causing renal pathology. Excessive leakage of protein appears to overwhelm the normal reuptake process of the proximal tubule cells. As a consequence the proximal tubule cells are stimulated to secrete several inflammatory cytokines including endothelin-1, monocyte chemoattractant protein-1 (MCP-1), and RANTES (Regulated upon Activation, Normal T Cell Expressed, and Secreted). These agents contribute to interstitial inflammation and fibrosis, which then leads to development of tubular dysfunction and eventually reduced ability to concentrate urine.8
Because proteinuria is a significant risk factor for reduced survival in cats with CKD, therapeutic strategies should be employed to minimize the proteinuria. Specific treatment should be instituted when the UPC is greater than 0.4 in cats without concurrent evidence of inflammatory or infectious lower urinary tract signs that could cause proteinuria. Angiotensin converting enzyme inhibitor therapy has been shown to reduce glomerular capillary pressure and to lower the UPC.4,9
The effect of dietary protein restriction on proteinuria in cats with stage II/III disease is not clear. Initial studies in feline remnant kidney models suggested a beneficial effect of protein restriction on the development of glomerular lesions.10,11 However, the results of these studies were confounded by alterations in both protein and energy intake. A subsequent study by Finco et al failed to demonstrate a benefit of protein restriction on renal lesions.12 The cats in Finco’s study developed borderline proteinuria (UPC 0.24 to 0.27); however, no significant difference in UPC was noted between any of the groups of cats. Based on studies in other species, it seems logical that restricting protein intake would limit feeding-related hyperfiltration. The patients most likely to benefit are those with UPC greater than 0.4. It is clear that further studies are required to evaluate the effect of protein restriction on proteinuria and disease progression in cats with naturally occurring CKD.
UREMIA
Every cat who is symptomatic for Stage III/IV CKD should benefit from a protein-restricted diet. At this stage of disease, the buildup of nitrogenous waste products reaches a level at which appetite is affected and irritation of the mucosal membranes with nausea and vomiting occurs. Controlled reduction of nonessential protein results in decreased production of nitrogenous wastes with consequent amelioration or elimination of clinical signs, even though renal function may remain essentially unchanged. The minimal dietary protein requirements for cats with CKD are not known, but are presumed to be similar to the minimal protein requirements of normal cats (i.e., 3.97 to 4.96 g/kg/day). However, this degree of restriction is necessary only in cats with profound uremia. For cats with documented azotemia and clinical signs, dietary protein should be reduced to approximately 20 to 25 per cent protein on a metabolizable energy (ME) basis. The dietary protein then should be adjusted to minimize excesses in azotemia while simultaneously avoiding excessive restriction because of the risk of protein malnutrition. Laboratory indicators of malnutrition include hypoalbuminemia, decreased blood urea nitrogen, hypocholesterolemia, anemia, and lymphopenia. However, alterations of these common laboratory indicators of malnutrition often are indistinguishable from those that can occur with CKD and/or concurrent disease. Furthermore, significant and often irreversible protein malnutrition has occurred prior to alterations in laboratory indicators. Clearly the dietary history and physical examination including body weight, body condition score, and cachexia score are more appropriate and sensitive indicators of protein malnutrition. The initial loss of lean body mass can be subtle and usually is first noted in the epaxial, gluteal, scapular, or temporal muscles. A subjective cachexia scoring system will facilitate the identification of those patients either with cachexia or at risk of impending cachexia (Table 14-2). If evidence of protein malnutrition occurs, dietary protein should be increased gradually until these abnormalities are corrected.
Table 14-2 Cachexia Scoring System
| Cachexia Score | Description |
|---|---|
| 0 | Good muscle tone with no evidence of muscle wasting |
| 1 | Early, mild muscle wasting, especially in the hindquarters and lumbar region |
| 2 | Moderate muscle wasting apparent in all muscle groups |
| 3 | Marked muscle wasting as evidenced by atrophy of all muscle groups |
| 4 | Severe muscle wasting |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree