CHAPTER 20 Exocrine Pancreatic Insufficiency
Exocrine pancreatic insufficiency (EPI) is a condition caused by insufficient synthesis and secretion of pancreatic digestive enzymes from the exocrine portion of the pancreas. As such, EPI is a functional diagnosis, rather than an etiological or morphological diagnosis. This means that the condition has two components: (1) a decreased synthesis and secretion of pancreatic enzymes from that which is normal, and (2) an amount of digestive enzymes insufficient for normal digestion of the dietary components. For example, there are reports of German Shepherd Dogs who have a severely decreased serum trypsin-like immunoreactivity concentration and no identifiable pancreatic tissue during exploratory laparotomy, but with no clinical signs of EPI.1 This is not surprising if one considers the physiology of digestion, which is a system characterized by great redundancy and exceptional functional reserve. For example, in cats dietary fat is digested by gastric and pancreatic lipase. The physiological importance of gastric lipase for fat digestion in cats is unknown, but it has been reported to be approximately 30 per cent in human beings and dogs.2 Of course, 30 per cent is an average, and there may be a wide range between individuals. In addition, digestibility of fat does not have to reach 100 per cent to avoid clinical signs of loose stools. Therefore pancreatic lipase may play a less crucial role in some patients, and some cats may need little to no exocrine pancreatic function to maintain normal stool quality. However, in general, some exocrine pancreatic function will be required to ensure physiological digestion.
EPIDEMIOLOGY
Disorders of the exocrine pancreas, and especially EPI, traditionally have been thought to occur less frequently in cats than in dogs or human beings. However, several studies of postmortem examinations have shown that significant pathological lesions are quite common in feline pancreata, and EPI is now recognized quite frequently in cats.3,4 Also, since the introduction of an assay (feline trypsin-like immunoreactivity [fTLI]) in 1995 for the diagnosis of feline EPI, the disease is now diagnosed much more frequently than before. For example, over a 10-year period (from the early 1980s to the early 1990s), of 180,648 cats entered into the Veterinary Medical Data Base (VMDB), located at that time at Purdue University, only 1027 cats (0.57 per cent) were recorded as diagnosed with disorders of the exocrine pancreas, and only 11 of those cats (0.006 per cent) were reported as being diagnosed with exocrine pancreatic insufficiency. In sharp contrast, over the last 5 years (2004 to 2008), the Gastrointestinal Laboratory at Texas A&M University assayed 84,523 serum samples for fTLI concentration and a total of 1342 samples had serum fTLI concentrations at or below 8.0 µg/L, which is diagnostic for feline EPI. Although these populations of cats are not the same, it is clear that the advent of this diagnostic test has led to a much more frequent diagnosis of EPI in cats.
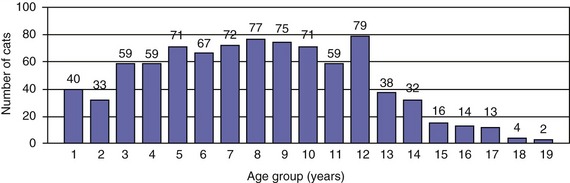
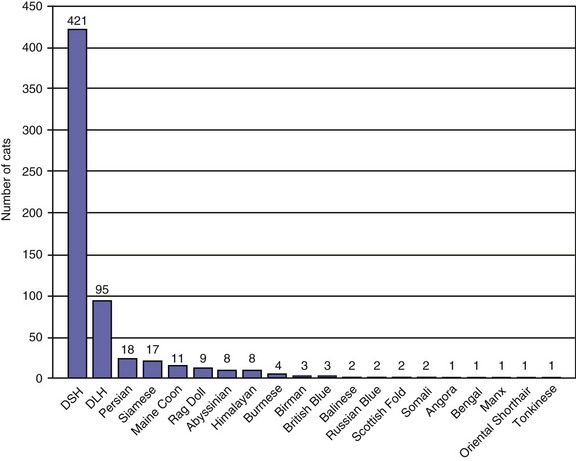
Traditionally, EPI has been thought of as a condition of older cats. However, the age distribution of 882 cats with a serum fTLI concentration at or below 8.0 µg/L assayed over the last 5 years showed an even distribution of ages (Figure 20-1). Seven kittens were 6 months of age or younger, and 40 cats were 1 year of age or younger. Cats who were older than 12 years of age were represented less frequently, most likely due to a decreasing number of cats who are older than 12 years of age in the general feline population. A breed predilection has not been reported for feline EPI. The author recently has evaluated breed characteristics for a group of 610 cats with a serum fTLI concentration at or below 8.0 µg/L, for whom breed information was available (Figure 20-2). Although the data were not compared to the general cat population, there was no apparent overrepresentation of any particular breed.
ETIOLOGY AND PATHOGENESIS
In theory, there are many potential causes of EPI, including pancreatic aplasia, pancreatic hypoplasia, pancreatic acinar atrophy (PAA), pressure atrophy due to pancreatic duct obstruction, and pancreatic destruction due to pancreatic inflammation. However, most of these theoretical causes of EPI have never been described in cats, and chronic pancreatitis is believed to be the most common cause of EPI in this species. Three cases of pancreatic acinar atrophy in cats have been mentioned in the literature, but none of these cases have been reported in detail.5 A few cases of EPI without preceding pancreatitis have been reported due to Eurytrema procyonis infestation.6 These pancreatic flukes attach themselves to the wall of the pancreatic ducts and may cause mucosal proliferation, periductal fibrosis, and duct obstruction, without parenchymal inflammatory infiltration.6 Adenocarcinomas of the exocrine pancreas or other neoplastic lesions in the area of the pancreatic duct or the duodenal papilla also can lead to obstruction of the pancreatic duct, followed by atrophy of acinar tissue; however, this also has never been reported in cats. Another rare cause of pancreatic duct obstruction may occur due to proximal duodenal resection, which occurs when the major duodenal papilla is damaged during surgery.5 Finally, congenital pancreatic hypoplasia or aplasia has never been reported in cats.
The clinical syndrome of EPI does not require deficiency of all pancreatic digestive enzymes. For example, isolated pancreatic lipase deficiency has been reported as a rare cause of EPI in human beings and also has been reported recently in one dog.7,8 However, isolated enzyme deficiency has not been reported previously in cats.
In human beings it has been shown that about 90 per cent of the functional reserve of the exocrine pancreas must be lost before clinical signs of EPI develop.9 Digestive enzymes of pancreatic acinar origin play an integral role in assimilation of all major food components. A lack of pancreatic digestive enzymes will lead to maldigestion. However, there also are disturbances in the transport mechanisms for monosaccharides and disaccharides, amino acids, and fatty acids, leading to malabsorption. The exact reason for this malabsorption is not known, but it has been speculated that the exocrine pancreas secretes trophic factors that act on the intestinal mucosa and that the secretion of these trophic factors is decreased in patients with EPI. The large amount of nutrients remaining in the intestinal lumen usually leads to loose, voluminous stools and steatorrhea. At the same time, the lack of nutrient assimilation will cause weight loss and may lead to vitamin deficiencies in some patients. Serum cobalamin (vitamin B12) concentrations are decreased in most cats with EPI.10 Serum folate concentrations in cats with EPI are either decreased, indicating concurrent small intestinal disease, or within the normal range, which is in contrast to canine and human patients with EPI, who often show increased serum folate concentrations. A cat with EPI with secondary vitamin K–responsive coagulopathy has been reported.11
In cases of EPI caused by chronic pancreatitis, destruction of pancreatic tissue may not be limited to the acinar cells, and concurrent diabetes mellitus may be observed.12,13 In fact, chronic pancreatitis has been recognized as an important cause of diabetes mellitus in human beings and dogs.13,14 Diabetes mellitus also has been reported in cats with EPI.12 Therefore cats with diabetes mellitus who have chronic loose stools should be evaluated for concurrent exocrine pancreatic disease. Also, because feline EPI in most cats is due to chronic pancreatitis, residual pancreatic inflammation may be present, and some cats may present with clinical signs that are compatible with both chronic pancreatitis and EPI.
CLINICAL PICTURE AND DIAGNOSIS
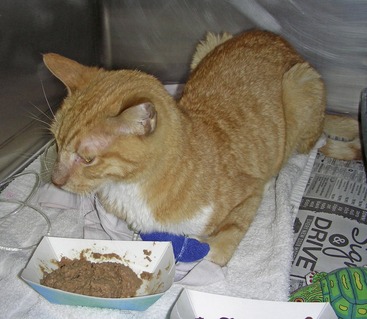
Clinical signs reported most commonly in cats with EPI are weight loss, polyphagia, and loose stools (Figure 20-3).12,15 Naturally, these clinical signs are nonspecific and also are seen in many cats with other disorders that are more common than EPI. Common causes of polyphagia in cats are hyperthyroidism, corticosteroid treatment, or diabetes mellitus. Common differential diagnoses for cats with weight loss are hyperthyroidism, dental and periodontal disease, chronic kidney disease (CKD), heart failure, neoplasia, and chronic intestinal disorders, such as inflammatory bowel disease. Finally, the most common disorders causing diarrhea are primary chronic intestinal disease or a variety of secondary disorders, such as hyperthyroidism, CKD, and liver failure.
The diarrhea is characterized by loose or semiformed voluminous stools, which may have a yellow to clay-colored appearance and may be quite malodorous (Figure 20-4). Some cats with EPI may develop watery diarrhea, but this is not common. The high fat content of the feces can lead to a greasy appearance of the haircoat, especially in the perianal and tail region. However, in a recent report of 20 cats with EPI, only one cat showed greasy soiling of the haircoat in the perineal region.16

Figure 20-4 A large stool sample from Theo, the cat in Figure 20-3, with untreated EPI. Note the typical loose consistency and the light brown color. Also, the stool sample appears to contain a lot of undigested food particles.
(Courtesy Dr. Kenneth Jones, Jones Animal Hospital, Santa Monica, CA.)
Recently, severe D-lactic acidosis in a cat with clinical signs of episodic generalized weakness, ataxia, and lethargy was shown to be secondary to EPI.17 The authors speculated that the D-lactic acidosis was due to massively increased bacterial fermentation in the intestinal lumen due to small intestinal bacterial overgrowth secondary to EPI.17 The D-lactic acidosis and associated clinical signs resolved in this cat after enzyme supplementation.17 (See Chapter 58 for a discussion on D-lactic acidosis and neurological signs.)
As mentioned previously, some cats with EPI have concurrent diabetes mellitus and may be presented with polydipsia and polyuria or even with acute complications of diabetic ketoacidosis. Also, patients may show residual signs of chronic pancreatitis, such as anorexia or abdominal discomfort. Similarly, many cats with chronic pancreatitis have concurrent inflammatory conditions of other abdominal organs, such as the intestines or the biliary system, and may show clinical signs associated with those inflammatory conditions (see Chapter 19).
Results of routine blood tests are within the normal range in most patients. Lymphopenia, lymphocytosis, neutrophilia, eosinophilia, and elevations of hepatic enzymes have been reported in a few affected cats.5 It is unclear from the literature whether these laboratory abnormalities are rare changes associated with EPI or, more likely, are associated with concurrent conditions, such as diabetes mellitus, inflammatory bowel disease, or chronic cholangitis.
Abdominal radiographs or ultrasound also do not show any specific changes in most cats with EPI. In one report, two cats with EPI showed an inhomogeneous pancreatic parenchyma and pancreatic nodules, but this has not been reported elsewhere.18 More common are radiographic and/or ultrasonographic changes due to concurrent conditions.
As mentioned above, EPI is a functional disease and thus requires a functional diagnosis. Several tests have been recommended to estimate exocrine pancreatic function in cats. The bentiromide absorption test (commonly known as PABA test), plasma turbidity, microscopic examination of feces for undigested fat, starch, or muscle fibers, and fecal proteolytic activity (FPA) all have been recommended for use in cats.5,15,19–21 With the exception of FPA, all of these tests are unreliable and/or impractical in cats and are not recommended. Fecal proteolytic activity can be determined by a variety of methods. The simplest method uses a piece of unexposed radiographic film. A small stool sample is placed on the film, and one would expect a clear halo to develop around the fecal sample in healthy cats. Unfortunately, this method is unreliable and should not be performed. The azocasein or azoalbumin methods are more reliable. However, FPA is very labile, and false-positive results can occur due to inappropriate sample handling. At least three stool samples from consecutive days should be evaluated. Also, feces need to be frozen immediately and shipped on ice in order to prevent loss of FPA in the samples.21 However, better diagnostic tests for feline EPI are now available, and FPA should only be used for species for which more current tests are not available.
Immunoassays for the measurement of fTLI concentration have been developed and validated analytically.10,22 Currently, there are only two assays for the measurement of serum fTLI available worldwide. The Gastrointestinal Laboratory at Texas A&M University offers a radioimmunoassay that uses purified feline cationic trypsin and a polyclonal rabbit antifeline cationic trypsin antiserum. This assay has been fully validated analytically.23 The current reference range for serum fTLI measured at the Gastrointestinal Laboratory is 12 to 82 µg/L, with values of 8 µg/L or lower being diagnostic for EPI. There also is an enzyme-linked immunosorbent assay (ELISA) for the measurement of fTLI available exclusively in Europe. To the author’s knowledge, the validation of this assay has not been described in the literature.
The fTLI assay has been used successfully to diagnose feline EPI for more than 10 years.10,24 In one report clinical data from the first 20 cats with serum fTLI concentrations at or below 8 µg/L were evaluated; 17 of these 20 cats showed compelling evidence of EPI and the remaining three cats had supportive evidence of EPI.16 All 20 cats in that study showed clinical signs compatible with EPI, and 17 cats showed a positive response to treatment. Two of the three cats who did not respond to therapy ultimately died, and a pancreatic biopsy strongly supported a diagnosis of EPI in both cases. The third cat who did not respond to therapy was vomiting and had decreased serum cobalamin and folate concentrations. Concurrent small intestinal disease or severe hypocobalaminemia may have impaired the therapeutic response to enzyme supplementation in this cat. On exploratory laparotomy, a small pancreas was noted by the surgeon, suggesting a diagnosis of EPI. However, a pancreatic biopsy was not collected. Two cats were 6 months old at the time of diagnosis. One cat showed poor growth and hair loss and responded to enzyme replacement therapy with weight gain and hair growth. The second cat was very thin and had a greasy haircoat. It also responded to enzyme replacement with weight gain. However, the cat was found dead 3 weeks after diagnosis and start of therapy, and a necropsy examination was not performed. Both cats responded with weight gain, but both still were immature at the time of diagnosis. Remission of some undiagnosed gastrointestinal disorder other than EPI may have led to growth and weight gain. It was strongly suspected that these three latter cats also had EPI, but evidence was not considered conclusive. Even if none of these three cats would have had EPI with false-positive test results, the specificity of serum fTLI concentration for EPI would still be 85 per cent. Therefore it was concluded that serum fTLI concentration is a specific test for EPI in cats.
Recently, it was shown that decreased renal function has a significant impact on serum fTLI concentrations and cats with renal failure may have falsely increased serum fTLI concentrations.25 Therefore evaluation of serum fTLI concentrations in azotemic cats may obscure a diagnosis of feline EPI. However, no patient has been identified to date in whom a diagnosis of EPI was missed because of renal failure. The author would suggest reevaluating serum fTLI concentrations in azotemic cats who have a borderline fTLI concentration and in whom an alternative diagnosis for loose stools and weight loss can not be identified. In contrast to dogs, serum fTLI did not increase significantly after feeding of healthy cats.26 However, this was not studied in cats with suspected EPI. Therefore the current recommendation is to withhold food whenever possible from cats for 12 hours before collecting a sample for measurement of serum fTLI concentration.
Most cats with EPI have severe serum hypocobalaminemia. In one study of 11 cats with EPI, serum cobalamin concentration was undetectable in 10 cats and subnormal in the remaining cat.10 Because hypocobalaminemia can lead to various gastrointestinal and systemic effects and also can result in treatment failure in cats with EPI, the author recommends that serum cobalamin and folate concentrations should be measured in every cat suspected of having EPI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree