Chapter 37. Nutrition and Mobility
It has been reported that almost one fourth of dogs visiting veterinary practices are diagnosed with musculoskeletal disorders. 1 Of these cases, 70% involved mobility or lameness issues of the appendicular skeleton. The incidence in dogs that are less than 1 year of age is about 22%, and more than 90% of these cases are thought to be influenced by nutritional factors. 2 Developmental skeletal diseases are most prevalent in the large and giant breeds, and their onset is usually associated with periods of rapid growth. The most common of these disorders are canine hip dysplasia (CHD), several forms of osteochondrosis, hypertrophic osteodystrophy (HOD) (also called metaphyseal osteopathy), and panosteitis. Congenital disorders that affect mobility include medial patellar luxation and Legg-Calvé-Perthes disease. Other causes of lameness and impaired mobility include ruptured cruciate, infectious arthritis, and rheumatoid arthritis. Finally, osteoarthritis (OA) is a common disorder in middle-aged and older pets and eventually develops with almost all forms of musculoskeletal disease. Free-choice feeding of diets that are nutrient- and energy-dense or supplementation with certain nutrients during growth are important factors in the etiology of developmental skeletal disease. In addition, nutritional management, along with antiinflammatory and pain medication, plays a critical role in alleviating clinical signs and supporting joint heath in pets affected with OA.
GENERAL CONSIDERATIONS
The musculoskeletal system is composed primarily of muscle, ligaments, tendons, cartilage, and bone. It provides structural support for multiple organ and metabolic functions as well as locomotion, mastication, and respiration. Because the musculoskeletal system is integrated with the cardiovascular, respiratory, neurologic, hemolymphatic, digestive, and endocrine systems, primary disorders of any of these interrelated systems can directly affect the musculoskeletal system. Therefore, when evaluating any lameness issue, other body functions and systems should also be considered.
Musculoskeletal dysfunction of the appendicular skeleton can involve one or more limbs. There can be muscle, tendon, joint, or bone involvement in one or more limbs. There may be swelling, pain, weakness, or atrophy present in muscles or tendons. Similarly, evaluation of affected joints may reveal swelling, heat, pain, crepitation, luxation or subluxation, and either increased or decreased range of motion. The bone itself may be involved. Fractures, bony enlargement, abnormal conformation, growth disturbances, and increased or decreased density seen on radiographs are all indictors that a bone may be the primary source of lameness. In all cases, a thorough veterinary examination and medical history are necessary for diagnosis of musculoskeletal disease.
DEVELOPMENTAL SKELETAL DISORDERS
Canine Hip Dysplasia
CHD is a biomechanical disease of the coxofemoral joint, characterized by incongruity between surfaces of the head of the femur and the acetabulum. Over time, this subluxation causes remodeling of the joint, typified by shallowing of the acetabulum, flattening of the femoral head, and development of OA. The degree of subluxation and joint laxity determines the degree of femoral head remodeling and development of degenerative joint disease over the dog’s lifetime (Figure 37-1). 3 Together, joint laxity (which becomes evident after 2 months of age) and OA, which can become evident as early as 4 months of age or be delayed for years, determine the severity of the disease in an individual. Affected dogs range from being asymptomatic throughout life to being severely crippled at a young age.
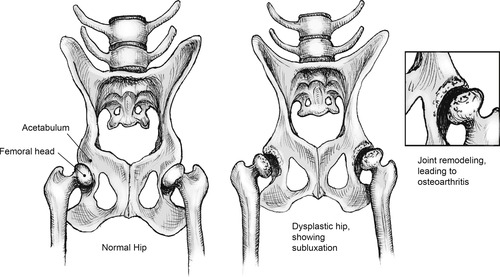 |
| Figure 37-1 |
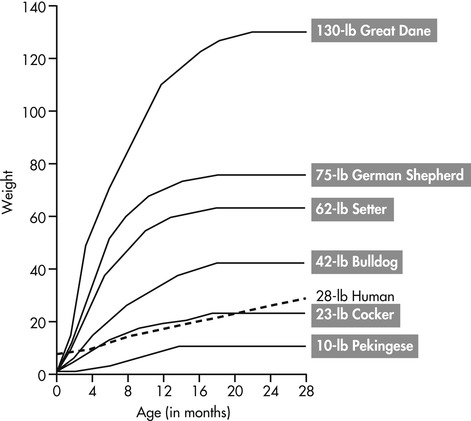
CHD is the most frequently encountered orthopedic disease in veterinary medicine. It has a multifactorial etiology, involving both a strong genetic component and a number of potential environmental factors. 4 Although all dogs are susceptible, breeds that have an increased risk of developing CHD include the Bulldog, Pug, Saint Bernard, Clumber Spaniel, Boykin Spaniel, and Bloodhound. 5 The prevalence of CHD in some breeds may be higher than 70%. 6 Of the environmental influences, diet and growth rate are believed to be most important. A dog’s rate of growth during the period from 3 months to 8 months of age is critical in the development of CHD (Figure 37-2). Dogs demonstrating a high rate of growth and excessive weight gain during this period have a higher frequency and more severe degenerative changes than do dogs growing at rates equal to or below their breed standard. 7 Additionally, excessive external muscular forces acting on the hip joint from strenuous or excessive exercise during periods of growth may prevent the femoral head from remaining in close contact with the acetabulum during development.
Dogs with severe hip dysplasia may show clinical signs of the disease when they are less than a year of age, but signs more typically appear in middle-aged dogs. Depending on the degree of CHD, obvious signs of OA may not be observed until the dog is middle aged or older because the hip joint develops more articular cartilage abnormalities as animals age. Clinically, dogs with hip dysplasia have difficulty rising from a lying or sitting position especially after exercise. They may move both legs together when running or loping and appear to “bunny-hop.” There may be pain associated with palpation of the joint on physical examination or even a notable laxity within the joint. If chronic, there may also be atrophy or muscle wasting of the pelvic and hind-limb muscles.
Canine hip dysplasia (CHD) is a developmental skeletal disease characterized by incongruity between surfaces of the head of the femur and the acetabulum. Over time, this subluxation causes remodeling of the joint and development of osteoarthritis. Large and giant breeds are more susceptible to CHD, and risk is increased if a dog experiences a high rate of growth and excessive weight gain during development.
Osteochondrosis
Osteochondrosis is characterized by a focal disruption in endochondral ossification, which causes impaired maturation of epiphyseal cartilage. 8 It can occur in both the physis and epiphysis of growth cartilage and at multiple points throughout the skeleton. In growing dogs, osteochondrosis of the articular epiphyseal cartilage most commonly occurs in the shoulder, stifle, hock, and elbow. Clinical signs include pain, lameness, and occasionally swelling around the affected joint. One or multiple joints may be affected, and signs typically appear when dogs are between 5 and 9 months of age. Subsequent to the development of osteochondrosis, acute inflammatory joint disease or OA develops when the cartilage joint surface is disrupted. A common manifestation of osteochondrosis is osteochondritis dissecans. This occurs when a segment of articular (joint) cartilage is separated from the underlying bone and subchondral bone is exposed to synovial fluid. If this piece of cartilage breaks off into the joint, it is commonly referred to as a “joint mouse” and typically requires surgical removal. As with CHD, the etiology of osteochondrosis appears to be multifactorial. Identified risk factors include age, sex, breed, rapid growth rate, and excessive weight gain, and possibly nutrient excesses (particularly of calcium). 9. and 10. Breeds that demonstrate the highest incidence of this disorder include the Bernese Mountain Dog, Great Dane, Labrador Retriever, Golden Retriever, Newfoundland, and Rottweiler. 11. and 12.
Hypertrophic Osteodystrophy
HOD occurs primarily in the large and giant breeds of dogs and is characterized by excessive bone deposition and retarded bone resorption. Breeds showing a high incidence include Great Danes, Saint Bernards, Boxers, Chesapeake Bay Retrievers, Irish Setters, German Shepherd Dogs, and Labrador Retrievers. 12 The distal ulna, radius, and tibia are most commonly affected. Radiographically, an irregular, translucent zone initially appears in the metaphysis and is separated from the growth plate by an excessively dense band of bone. As the disease progresses, additional bone is deposited outside of the periosteum, and soft-tissue swelling and subperiosteal hemorrhages develop around affected metaphyseal areas. Signs include acute pain and swelling, lameness, intermittent pyrexia, and occasional anorexia. The observed swelling is due to both fibrous thickening of the periosteum and the deposition of new periosteal bone. HOD primarily affects dogs that are growing rapidly, and the initial signs are typically seen when dogs are between 3 months and 6 months of age.
Panosteitis
Panosteitis is a common cause of lameness in young, large-breed dogs and is typically diagnosed when dogs are between 5 and 12 months of age. Males are more frequently affected than females, and Great Pyrenees, German Shepherd Dogs, Mastiffs, and Basset Hounds are at increased risk. 12 Panosteitis is characterized by generalized inflammation of the long bones, specifically the humerus, radius, ulna, femur, and tibia. 13 In more than half of the reported cases, the disorder moves from leg to leg, eventually affecting almost all of the long bones. Affected dogs show a sudden onset of moderate to severe lameness, with no history of injury or trauma. A defining characteristic of panosteitis is its tendency to shift, moving from one limb to another over a period of several months or more. Because of this, panosteitis is often erroneously referred to as “growing pains” in young dogs, as it is often seen during periods of rapid growth. Affected puppies may show a loss of appetite and transient fever during acute episodes. Although dogs may show extreme lameness and pain upon palpation of the affected limbs, panosteitis is a self-limiting disorder in the vast majority of dogs. Still, this disorder can be very concerning to owners because some dogs show intermittent lameness for up to 18 months or more.
Nutritional Factors Influencing Developmental Skeletal Diseases
Most forms of developmental skeletal disease are influenced by genetics and are considered to be polygenetic diseases. However, heredity cannot fully explain their occurrence, and phenotypic expression depends upon a variety of environmental influences. For example, although a limited number of breeds have been studied, heritability coefficients for CHD are estimated to be between 0.1 and 0.4. 14.15. and 16. This means that 60% or more of the factors that influence the phenotypic expression of CHD are environmental in nature. Although it is difficult to identify all of the external components involved in these disorders, nutrition and rate of growth play an important role. A number of nutrients have been examined, including energy, protein, vitamin C, fat, carbohydrate, and calcium. The data indicate that the most important nutritional factors in the development of skeletal disease in dogs are excess caloric intake and excessive intake of calcium during growth.
EXCESS CALORIC INTAKE
Excess energy intake during growth commonly occurs as a result of feeding a high-quality, growth diet to a young dog on a free-choice basis or feeding excess quantities of food on a portion-controlled basis. Some owners believe that puppies should be kept “plump” in appearance, and that a rotund puppy is a healthy puppy. However, when puppies are fed excessive amounts of a balanced diet, growth rate is maximized before excess weight in the form of fat is gained. As a result, a growing dog that appears to be slightly overweight is usually growing at a maximal rate. Studies with dogs, humans, and other species have shown that the consumption of excess calories resulting in a maximal or an above-average growth rate is not compatible with optimal and healthy skeletal development. 17. and 18.
The first study to demonstrate the relationship between overnutrition, rapid growth, and developmental skeletal disease was conducted with growing Great Danes. 19 Two groups of growing puppies were fed a highly palatable, energy-dense food throughout growth. The first group was fed free-choice, and the second group was fed amounts of food that were restricted to two thirds of the intake of the first group. The dogs that were fed free-choice grew significantly faster than did the dogs fed restricted amounts of food, and bone tissue was significantly affected by the rapid growth rate. A variety of skeletal abnormalities were observed in the dogs that were fed free-choice, including enlargement of the costochondral junctions and the epiphyseal-metaphyseal regions of long bones, hyperextension of the carpus, and sinking of the metacarpophalangeal and metatarsophalangeal joints. These changes were associated with pain and varying degrees of lameness. It was concluded that generalized overnutrition, in the form of excess energy, protein, calcium, and phosphorus, caused increased growth rate in these dogs that contributed to abnormal skeletal development.
Subsequently, other studies were conducted with growing dogs of several large breeds, including German Shepherd Dogs, Golden Retrievers, and Labrador Retrievers. In one study, a group of puppies that had high parental frequencies of CHD were examined. The incidence and severity of dysplasia was greater in puppies that had rapid growth rates as a result of increased caloric intake, when compared with puppies that were fed restricted amounts of food. 20 Similarly, when a group of puppies was hand-reared at a reduced rate of growth, they developed a very low incidence of hip dysplasia. 21. and 22. In contrast, a control group that was fed to allow a much higher growth rate showed a very high incidence of hip dysplasia.
A well-controlled longitudinal study with a group of 48 Labrador Retrievers found that dogs fed 25% less food than dogs fed free-choice, starting after weaning and continuing throughout life, had significantly less hip joint laxity at 30 weeks of age and a significantly lower incidence of CHD. 18. and 23. Of the dogs in the free-choice group, 16 of 24 developed CHD by 2 years of age, compared with 7 of the 24 dogs in the limited-feeding group. OA was observed in the hips of 7 of the 24 free-choice dogs by the time they were 1 year old, while none of the limited-feeding dogs showed OA at 1 year. 24 By 5 years of age, 12 of the 23 remaining free-choice dogs (52%) and only 3 of the 23 limited-feeding dogs (13%) had radiographic signs of hip OA, and the degree of OA was more severe in free-choice dogs than in the restricted-feeding dogs. Additional evaluations showed that arthritic changes to hips and shoulders increased linearly (rather than bimodally) throughout life, and these changes were more frequent, occurred earlier, and were more severe in dogs fed free-choice compared with dogs fed restricted amounts of food. 25. and 26. Finally, the dogs fed free-choice weighed more and had higher body condition score values during growth and throughout life; a significant and positive correlation was found between body weight and the presence and severity of OA. 24
The skeletal system of dogs is most susceptible to physical and metabolic stressors during the first 12 months of life. It is theorized that abnormal skeletal development during periods of rapid growth results from overloading the growing skeleton with prematurely increased muscle mass and body weight. 27 This theory is supported by the fact that rapidly growing male dogs of the large and giant breeds are more frequently affected than are smaller females. 28 There also appears to be a correlation between increasing body size and the occurrence of the lesions associated with osteochondrosis. 29 A comparison between the bones of large and small breeds of dogs during periods of rapid growth shows that the bones of large breeds are relatively less dense than are the bones of small breeds at similar stages of development. Bones of large breeds have a thinner cortex, larger medullary cavity, and a less dense spongiosa. 29. and 30. It is possible that the bones of large dogs during growth are not as strong as those of smaller dogs during the same period. This may be the basis for the genetic predispositions toward skeletal abnormalities in the large and giant breeds. Recent evidence suggests that mechanical stress on the joints caused by excessive weight during growth and throughout life also contributes to the development of OA. 24
In addition to weight-related mechanical stressors, overnutrition has direct effects that negatively influence skeletal development. Studies with Great Danes have shown that overnutrition stimulates accelerated skeletal growth. 29 Great Dane puppies were fed a diet formulated for growth from weaning to 6 months of age. Puppies in one group were fed free-choice (ad libitum), and puppies in the second group were restricted to 70% to 80% of the amount consumed by the first group. As in previous studies, dogs fed ad libitum weighed significantly more than the restricted group at 6 months of age. Bone measurement data showed that accelerated skeletal growth, in the form of increased size and volume of bone, contributed significantly to the increased weight. The male dogs that experienced overnutrition also showed an increased rate of bone remodeling, resulting in enlarged bones with relatively low densities and low resistance to the greater weight they were required to bear. It appears that if a large dog is allowed to attain its maximal growth rate by consuming excess amounts of a balanced diet, the accelerated growth rate creates a rapidly growing skeleton that is less strong and less able to withstand the biomechanical stresses of the greater muscle mass and body weight that are put on it. Not only do these dogs weigh more, but their bones are less able to handle the added weight. The end result is the development of aberrations in ossification, damage to developing cartilage and growth plates, and premature closure of growth plates. Most often this manifests as osteochondrosis, but these changes may also be involved in the onset of several other developmental skeletal diseases.
Excess energy intake during growth is a significant risk factor for developmental skeletal disease in dogs. This most commonly occurs as a result of feeding a high-quality, growth diet to a young dog on a free-choice basis or feeding excess quantities of food on a portion-controlled basis. The resulting rapid growth rate is not compatible with healthy skeletal development because of mechanical stresses placed on developing bones and changes in bone growth.
Feeding for Optimal Growth Rate
The final skeletal height of a dog is strongly influenced by genetics. Providing adequate, but not excessive, amounts of a balanced diet enables an animal to achieve its potential size but at a slower rate than if excess food is provided. Feeding an energy-dense, nutrient-balanced food at a level that promotes a high rate of growth decreases the time it takes the dog to attain adult size and can contribute to abnormal skeletal development. Conversely, feeding restricted amounts of a balanced food, to achieve a slower growth rate, results in an animal of the same size, but at a later point in time. Allowing the skeleton to develop slowly, and feeding to maintain a lean body condition, eliminates the biomechanical stresses of excess weight and the changes in bone development that are associated with rapid growth and overweight body conditions.
The best way to attain a moderate growth rate is to strictly monitor body weight during growth and to feed to attain a lean body condition (Box 37-1; see Chapter 28, Figure 28-2, p. 324). The chief contributor to rapid growth and weight gain is dietary energy. Because fat is much more energy dense than protein or carbohydrate, high-fat diets contribute significantly to excess energy intake. Several commercial dog foods are specifically formulated for large-breed puppies. These foods contain slightly lower fat and energy than typical puppy foods and have a protein concentration that is balanced to maintain an appropriate protein-to-energy ratio. Although dietary protein was once identified as a potential contributor to skeletal abnormalities, studies have shown that dietary protein level is not a risk factor for the development of skeletal disorders in growing dogs. 31 A general recommendation is to select a food that has a moderately reduced energy content (between 3.5 and 3.8 kilocalories [kcal]/gram [g] of dry food) compared with typical puppy foods, and between 12% and 16% fat. The food should contain a minimum of 22% high-quality protein, with an optimum level of between 26% and 28% (23% to 26% of calories). 32 Calcium levels should be between 0.8% and 1.2% (see pp. 499-500).
BOX 37-1
Select a complete and balanced dog food that has been formulated for growth in large and giant breeds.
Feed this food throughout the first 1 to 2 years of life.
Use a portion-controlled feeding regimen and carefully measure the amount of food that is fed each day.
Provide an amount of food that will support an average rate of growth for a dog’s breed.
Provide an amount of food that will maintain a lean body condition throughout growth.
Strictly monitor weight gain and body condition until the dog reaches maturity.
Do not supplement the diet with minerals, vitamins, or additional foods.
Large- and giant-breed dogs should always be fed on a portion-controlled basis during growth. Free-choice feeding is not recommended because it increases the risk of overconsumption and is not conducive to monitoring daily intake and controlling rate of growth. In addition, ad libitum feeding appears to affect several of the hormonal regulatory systems of growth. 33 Circulating levels of insulin-like growth factor-1, thyroxine (T 4), and triiodothyronine (T 3) were found to be higher in growing dogs that were fed ad libitum when compared with levels in dogs that were fed on a portion-controlled basis. It is possible that ad libitum feeding promotes the metabolic processes that support rapid growth rate. Portion-controlled feeding allows the owner to feed an amount of food that maintains optimum growth rate and body condition and to gradually adjust the dog’s intake as energy needs change during growth.
The amount to feed can be estimated from guidelines provided on the food’s package and then adjusted to attain ideal body condition (see Chapter 28, Figure 28-2, p. 324). Because growing large- and giant-breed dogs have very steep growth curves, their intake requirements can change dramatically over short periods of time. Therefore puppies should be weighed and evaluated at least once every 2 weeks. Young puppies should be fed three to four meals per day until they are 4 months old and two meals per day thereafter. A well-formulated, breed-size–specific growth diet should be fed throughout the dog’s growth period. For large and giant breeds, this corresponds to the first 12 to 24 months of life, depending upon the dog’s breed and adult size.
The best way to attain a moderate growth rate is to strictly monitor dogs’ body weight during growth and to feed to attain a lean body condition. A growth food that contains slightly reduced fat and energy should be selected for large- and giant-breed puppies. Feeding on a portion-controlled basis is also recommended because this allows the owner to feed an amount of food that maintains optimum growth rate and body condition and to gradually adjust the dog’s intake as energy needs change.
Excess Calcium Intake and Skeletal Disease
Calcium is a nutrient that is commonly supplemented to the diets of dogs and, less commonly, to the diets of cats. The reason most often cited for calcium supplementation relates to its essential role in normal skeletal growth and development. Supplements such as dicalcium phosphate and bone meal are added to a growing dog’s diet during growth spurts or when problems such as hyperextension of the carpus or sinking of the metacarpophalangeal joints occur. Some breeders encourage all of their puppy buyers to routinely supplement their puppy’s diet with calcium during the entire first year of life as a prophylactic measure. Some believe that calcium supplementation is not only necessary for proper bone development but that it also prevents the development of certain skeletal disorders. Regardless of the good intentions, there are potential risks when excessively high levels of calcium are either included in the diet itself or added to an adequate and balanced diet.
Research has shown that normal growth in puppies can be supported by a calcium intake of 0.37% available calcium or 0.6% total calcium. 34 The Association of American Feed Control Officials’ (AAFCO’s) Nutrient Profiles for dog foods sets minimum levels for calcium of 0.8% for growth and reproduction and 0.5% for adult maintenance. The profile also mandates a maximum level of 2.5% calcium in all dog foods. This maximum level was included because published data show that excess calcium during growth may contribute to abnormal skeletal development and increase a dog’s risk of skeletal disease.
A series of studies were conducted in the 1980s to determine the effect of varying levels of dietary calcium upon the occurrence of developmental skeletal disease in growing Great Danes. 35. and 36. An experimental diet was formulated that met the recommendations of the 1974 National Research Council’s (NRC’s) Nutrient Requirements for Dogs. Both the control group and the experimental group of dogs received this food throughout growth. In addition, the experimental group received calcium carbonate supplementation to achieve a level of 3.3% in the diet, three times the amount recommended by the NRC at that time. 37. and 38. Dogs that were fed excessive calcium during growth developed chronic hypercalcemia and hypophosphatemia. Skeletal differences between the control and experimental dogs included a higher percentage of total bone volume, retarded bone maturation, retarded bone remodeling, and a decreased number of osteoclasts (bone resorption cells) in the dogs receiving calcium supplementation. This group also showed a higher incidence and severity of cartilage irregularities associated with osteochondrosis at the distal and proximal humeral cartilages. Clinically, calcium-supplemented dogs exhibited retained cartilage cones, severe lateral deviation of the feet, and radius curvus syndrome.
When plasma calcium levels increase, the hormone calcitonin is secreted from the thyroid gland. Calcitonin functions to lower plasma calcium to normal levels; it exerts this effect by decreasing the activity of osteoclasts, which leads to decreased bone resorption and slowed cartilage maturation. 19. and 35. This is a normal hormonal response to both eating and calcium influx, and calcitonin functions along with parathyroid hormone (PTH) to closely regulate calcium homeostasis. However, the chronic elevation of calcitonin suppresses bone resorption for an abnormal period of time, resulting in a gradual thickening and increased density of cortical bone. For example, when growing Beagles were supplemented with 2.3 g of calcium per day for 2 months, the thyroid glands of supplemented dogs contained significantly increased proportions of calcitonin-producing C cells and decreased proportions of thyroid follicles, when compared with those of the control dogs. 39 High dietary calcium intake caused thyroid C-cell hyperplasia, which would suggest the occurrence of chronic hypercalcitoninism in these dogs. Additionally, electron microscopy of the thyroid C cells of dogs fed excess calories, protein, and calcium showed that these cells were releasing larger amounts of calcitonin than were the C cells of dogs fed restricted diets. In growing dogs, these changes interfere with normal bone remodeling and development. The deposition of excessive subperiosteal bone that results may cause the clinical signs of HOD and Wobbler syndrome, and the chronic effects of calcitonin on cartilage maturation may result in the eventual detachment of the articular cartilage that is seen in osteochondrosis.
A more recent study, again with Great Danes, fed the same diets described previously plus a third experimental diet that contained additional phosphorus to provide a balanced calcium-to-phosphorus ratio. 40 Puppies in this study were fed the high calcium diets during the period of most rapid growth, between 6 and 17 weeks of age. At 17 weeks, all of the puppies were switched to the control diet, which contained normal calcium and phosphorus levels (1.04% and 0.82%, respectively). Puppies fed the high calcium-only diet developed hypercalcemia and hypophosphatemia that persisted throughout the experimental diet feeding period. Puppies fed the food containing elevated calcium and phosphorus had normal serum calcium values during this period and slightly lowered plasma phosphorus levels. Although both groups of puppies that were fed excessive calcium showed skeletal disturbances during the feeding period, changes were more severe in puppies fed excessive calcium without phosphorus. This difference was attributed to the hypercalcemia and subsequent reduction in PTH secretion, leading to hypophosphatemia. This study also found that normalizing dietary calcium levels after 17 weeks of age corrected some skeletal abnormalities, but joint changes that were consistent with osteochondrosis still developed in the puppies that were fed the diet containing high calcium without added phosphorus.
To further examine the effects of elevated calcium when a normal calcium to phosphorus ratio is maintained, Great Dane puppies were fed diets containing three levels of calcium (0.48%, 0.8%, and 2.7%). 41 Each diet contained sufficient phosphorus to provide a consistent calcium-to-phosphorus ratio of 1.2:1.0. To control for other factors that can affect skeletal development, the experimental diets had reduced caloric density and contained 26% protein and 14% fat. Puppies were assigned to the three diets at the age of weaning, and they were fed the experimental diets for 18 months. Even when balanced with phosphorus, increasing dietary calcium had a rapid and direct effect on the amount of mineral deposited in maturing bones. Bone mineral content and bone density were positively correlated with dietary calcium and phosphorus beginning almost immediately after weaning and continuing until the dogs were 6 months old. Six of the 15 dogs that were fed the highest level of calcium (2.7%) showed clinical signs of lameness during the first 6 months of the study, and three of these dogs showed clinical signs of HOD. After 6 months, the bone density differences seen between the high-, medium-, and low-calcium groups gradually diminished and were not apparent by the time that the dogs were 12 months old (Table 37-1). Similar to the results of Schoenmakers et al., 40 dogs fed high amounts of calcium that were balanced with phosphorus did not develop hypercalcemia. Conversely, dogs fed the highest calcium diet had low total and ionized serum calcium values when they were between 4 and 7 months of age. This decrease was not observed in dogs fed the low- and medium-level calcium diets. It is likely that these values are a result of increased deposition of circulating calcium into developing bone, possibly in response to chronically increased calcitonin levels. The normalization of bone composition between the three groups after 6 months of age may reflect the natural maturation of the dogs’ regulatory mechanisms that control calcium absorption and excretion (see below).
| a,b,c, Values within a row with different superscripts differ ( P<0.05). | |||
| A ge ( months) | L ow calcium (g) | M oderate calcium (g) | H igh calcium (g) |
|---|---|---|---|
| 2 | 77.55b | 83.27b | 110.38a |
| 6 | 905.06b | 1066.63c | 1201.87c |
| 12 | 1768.64b | 1916.68a,b | 2072.70a |
| 14 | 2031.21 | 2069.72 | 2132.20 |
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree