Chapter 19 Nuclear Medicine
Scintigraphy and Other Imaging Modalities: Where do They Fit?
Recent advances in imaging such as digital radiography (Chapter 15), computed tomography (CT; Chapter 20) and MRI (Chapter 21) have led to questions about the role of scintigraphy in lameness diagnosis. We are often asked questions such as, “Would you use scintigraphy or MRI for diagnosis?” and “Should I put MRI or scintigraphy into my practice?” We would not choose to be without the ability to use scintigraphy in lameness diagnosis. We believe that the combined and concurrent use of scintigraphy and MRI yields the most useful and relevant information and that seldom, given strict case selection, is information redundant. At the origin of the suspensory ligament in the proximal aspect of the third metacarpal and metatarsal bones, a complex area from which pain commonly originates, a combination of imaging modalities is most useful to characterize soft tissue and bony injury. Likewise, lameness abolished with palmar digital analgesia is complex, and the concurrent use of advanced imaging modalities provides complementary information. Nuclear scintigraphy has proved useful in the investigation of foot pain, specifically from the podotrochlear apparatus, and has helped determine clinical significance of lesions discernible with MRI, because a positive correlation between scintigraphy and MRI grades in lesions of the navicular bone was found.8,9 We believe that both scintigraphy and MRI are critical for addressing complex lameness questions.
Radioisotopes and Radiopharmaceuticals
Accumulation of 99mTc-MDP is not simply the result of changes in local blood flow, although blood flow is likely increased in sites of actively remodeling bone. Although increased blood flow does not significantly affect a bone scan,10 adequate blood flow is necessary to deliver radiopharmaceutical to available binding sites in bone. Poor correlation was found between perfusion index and radiopharmaceutical uptake (RU) in delayed images when evaluating people undergoing distraction osteogenesis.17 This study suggested that blood flow is not closely linked to bone metabolism and that delayed images most accurately predicted osteogenesis.17 Rather than measuring blood flow, delayed images reflect changes in bone metabolism. When three-phase bone scintigraphy was used in people with osteonecrosis of the jaw secondary to bisphosphonate administration, delayed images were all positive, but increased perfusion (and increased blood pool) was found in 9 of 12 patients.18 A three-phase bone scan can detect increased perfusion and soft-tissue inflammation if present, but it is metabolic activity of bone that influences delayed phase images. Decreased blood flow, caused by infarction or ischemia, can greatly affect a bone scan but is an unusual clinical problem (see discussion of photopenia, page 226). However, decreased peripheral blood flow in old horses or those imaged in cold weather or on days with high diurnal temperature change can adversely affect image quality (see discussion of poor-quality bone uptake, page 222).
Imaging Equipment
A reconditioned gamma camera is perfectly satisfactory, less expensive than new equipment, and durable, often being useful for at least 15 to 20 years or more, with proper maintenance. Gamma cameras are either large field of view (LFOV) or small field of view (SFOV), based simply on the size (Figures 19-1 to 19-3). LFOV cameras can be rectangular or circular in shape, but crystals for rectangular cameras are more costly. In adapting equipment for use in horses, an important consideration is how to move the camera safely and easily to the horse and vice versa. Most standard gantry (support structure of the gamma camera) designs are unsuitable for equine imaging, because good-quality images can be obtained only by having the body part close to the camera. Resolution is inversely proportional to the distance between the body part and camera. To quickly obtain dorsal and lateral, and lateral and plantar, images of the forelimbs and hindlimbs, respectively, the camera must be able to be lowered below floor level (see Figure 19-3). Alternatively, the horse must be positioned on an elevated platform. Limbs can be held manually near the camera, but this practice increases radiation exposure to personnel and should be avoided whenever possible. Suitable gantries are commercially available or can be custom made. A lead collimator is also required.
Image Acquisition
An important recent advancement is the ready availability of user-friendly computer programs to acquire, analyze, store, and process images. Systems based on Apple, Windows, and Unix are currently available and are straightforward. Motion correction software is the latest, exciting advance; it improves upper limb and axial skeleton image quality by negating the effects of motion and allowing higher count numbers than with conventional software (Figure 19-4). Modern software also allows postprocessing of images—for example, masking out the bladder, which might otherwise steal counts.
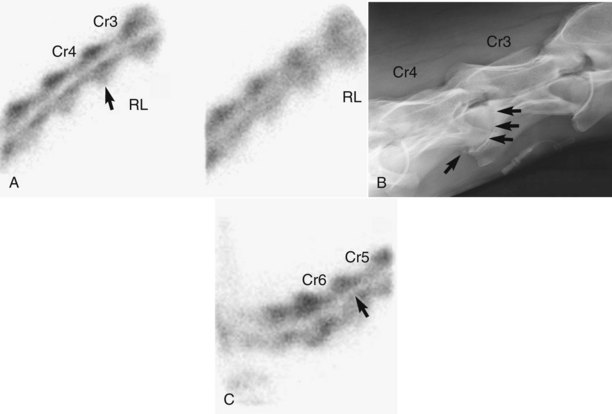
The number of images to obtain is based on the body part being examined, but usually at least two images are required. In some areas this may not be possible, but the clinician should be aware that false-negative results can occur. A dorsal scintigraphic image gives detailed information about only the dorsal aspect of the limb, and abnormal palmar regions might be missed. The image and information are not the same as depicted in a dorsopalmar radiographic image. Similarly, areas of IRU on the medial side of the limb might not be visible on a lateral image. Areas more distant from the camera contribute substantially less radiation than those closer because of the inverse square law. Bone interposed between a medial lesion and the camera can effectively shield the site. A lateral image of a right forelimb failed to reveal a fracture of the medial aspect of the distal phalanx (Figure 19-5). Radiographic examination and dorsal and solar scintigraphic images, however, revealed an incomplete fracture that we completely missed in the lateral scintigraphic image. Additional images may be necessary for accurate diagnosis.
We prefer to localize lameness, because this allows for detailed examination of a specific area. For instance, routine screening images of the front digit include lateral and dorsal delayed images, but in horses in which lameness is abolished using palmar digital analgesia, pool phase images and lateral, dorsal, solar, and occasionally medial, palmar, and flexed delayed images are obtained. Diagnostic accuracy can be improved by acquiring many different images, and pinpointing a lesion using scintigraphy can allow a focused radiographic, ultrasonographic, CT, or MRI study to be performed. Combined imaging can yield additional useful information.8,9 When augmenting the routine dorsal and plantar images of the metatarsophalangeal joint with flexed lateral and sometimes flexed dorsal and medial images, instead of saying, “There is IRU in the fetlock joint,” the diagnostician can say, “There is focal IRU involving the distal, plantarolateral aspect of the MtIII,” a much more accurate description (Figure 19-6).
Dynamic acquisition can be used before motion correction in delayed images or to evaluate blood flow. One- or 2-second per frame images are generated sequentially and can be evaluated individually or combined into a single composite image, with or without motion correction. First-pass angiography can be used to assess blood flow in the aorta, iliac, and femoral arteries33 in horses with suspected thromboembolism (Figure 19-7) or to assess blood flow in the distal limb.