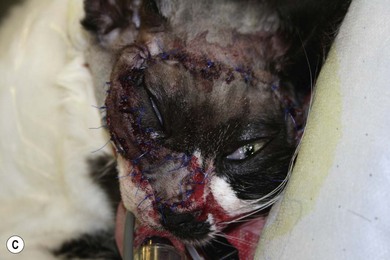
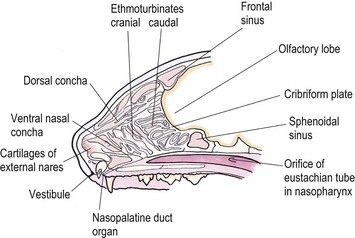
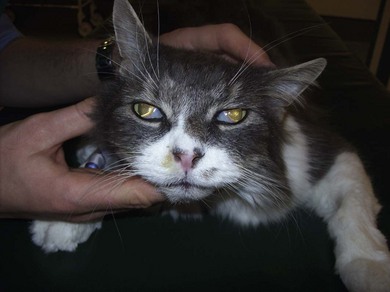
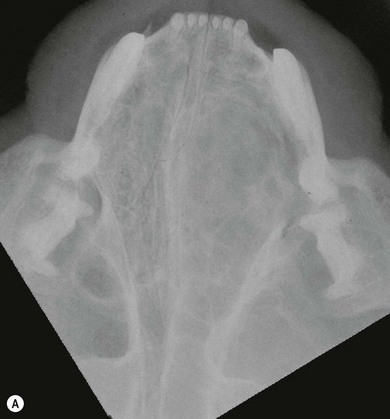
Chapter 54 The nose consists of the nasal planum, the nasal cavities or fossae divided by the nasal septum, and the choanae that mark the start of the nasopharynx (Fig. 54-1). The nasal fossae are divided by a dorsal concha (the extension of an endoturbinate), a curved shelf of bone originating from the ethmoidal crest. The dorsal concha (previously nasoturbinate) separates the dorsal cavity into the dorsal meatus and the middle meatus. Ventrally, the ventral nasal concha (previously maxilloturbinate) extends from the conchal crest and divides into several delicate bony scrolls. The concha divides the nasal cavity into the middle meatus and ventral meatus before forming the alar fold rostrally. Caudally, the ethmoturbinates extend from the midline ethmoidal plate to the cribriform plate; these ethmoid tubinates fill the presphenoid sinus and continue up into the frontal sinus. The ethmoturbinates can be divided into long, medially lying endoturbinates (usually seven in the cat) and smaller, more superficial ectoturbinates. The nasopharyngeal meatus is formed by the confluence of the caudal ends of the dorsal, middle, and ventral meatuses and runs to the choanae. The turbinates are covered by mucosa and act to direct the inspired air into the meatuses and humidify inspired air prior to passage to the lungs. They also act as a heat exchange mechanism and remove inhaled foreign material. Figure 54-1 The nasal cavity of the cat, showing the position of the ethmoturbinates, nasoturbinates and maxilloturbinates. Clinical signs of nasal disease include sneezing, stertor, nasal discharge, epiphora, epistaxis, inappetence, dyspnea, and facial swelling or distortion (Fig. 54-2). The clinical signs are often non-specific and may be associated with a variety of underlying diseases such as infectious rhinitis, inflammatory rhinitis, tumors (such as lymphoma or adenocarcinoma), foreign bodies, hypertension, or trauma. Increased upper respiratory tract noise and dyspnea are more commonly seen with neoplasia than foreign bodies or rhinitis.1 Figure 54-2 An eight-year-old female neutered domestic short-haired cat with a right-sided nasal discharge of five weeks’ duration. A nasal carcinoma was diagnosed after rhinoscopy and biopsy. Although history and clinical signs can be helpful in prioritizing differential diagnoses, unfortunately there is considerable overlap between most of the underlying diseases. Thus a careful diagnostic approach is essential, involving blood profiles (and ideally feline leukemia virus (FeLV)/feline immunodeficiency virus (FIV) status), imaging (including rhinoscopy if available), and histology. It can be difficult to obtain a representative sample of tissue for biopsy unless a mass is directly visualized on rhinoscopy and thus a histopathology report of lymphoplasmacytic rhinitis in a cat with a rapid onset of progressive clinical signs warrants a repeat biopsy. It is also worth considering that in approximately 50% of cases of rhinitis in cats a diagnosis is not achieved after workup. In a retrospective study of 75 cats, the overall diagnostic yield was 36%. However, in 16 out of 23 cats that underwent nasal imaging, rhinoscopy and nasal biopsy, a specific etiological diagnosis was obtained, demonstrating that if nasal disease is to be investigated the investigation needs to be thorough.2 The nose can be imaged with radiography, computed tomography, or magnetic resonance imaging (MRI). For radiography, general anesthesia is essential for optimal views. A complete radiographic examination of the nose would include a lateral and dorsoventral skull and a dorsoventral intra-oral view; the latter being the most useful view. The dorsoventral intra-oral view gives good detail of the nasal chonchae and septum but requires non-screen film and thus cannot be performed with digital radiography. If digital radiography is being used, a ventro 30° rostral-dorsocaudal view can be used. In cases with suspected sinus involvement, though the lateral view is useful, a rostrocaudal sky-line or ventral 10° ventro-caudodorsal oblique sinus view is helpful, although in some cats, particularly brachycephalic breeds, this can be a very difficult view to achieve and perfect. Radiographic abnormalities include soft tissue opacity, loss of turbinate detail, lucent foci, displacement of midline structures, soft tissue opacity of the frontal sinus, and facial deformity. Unfortunately most of these changes can be seen in both rhinitis and neoplastic disease. In one study of 64 cats, rhinitis was more frequently associated with a normal radiographic appearance while neoplasia more frequently caused displacement of midline structures, unilateral soft tissue opacity, unilateral loss of turbinate detail, and evidence of bone invasion.3 There was, however, considerable overlap between the two groups although none of the cats in the neoplastic group had normal radiographic changes. Computed tomography (CT) is better at localizing and determining the extent of nasal disease compared to radiography, but there is no difference in sensitivity of detection of the presence of nasal disease.4 Rhinoscopy allows direct visualization of the nasal cavity and biopsy (at least the first sample) to be guided. Some diseases such as nasal hamartomas, fungal granulomas, and foreign bodies can be treated appropriately with this technique. Retrograde rhinoscopy is performed first using a fiberoptic bronchoscope to examine the nasopharynx and choanae so that hemorrhage does not obscure the view. Anterior rhinoscopy is then performed with a 2.7 mm or 0.9 mm diameter (for smaller cats) forward-viewing rigid endoscope (for details on equipment and technique, see Chapter 9). Isotonic fluid can be used during rhinoscopy to flush away blood contamination and aid hemostasis. In one study of 41 cats with chronic nasal disease, a mass was evident rhinoscopically in the 19 cats with neoplasia.5 In feline nasal disease, cytologic analysis of samples from masses prepared by squash techniques can provide a relatively accurate diagnosis; agreement between cytologic and histologic diagnosis had a sensitivity of 0.94 and a specificity of 0.81 in one study of 30 cats.6 With brush sampling, the results are far less reliable: one study of 12 cases had only a 25% agreement between cytology and histology.7 The different results are probably due to the difficulty in locating a representative sample of tissue rather than superficial mucosa with the brush techniques as compared to the deeper sample obtained from a piece of abnormal tissue with the squash technique. Cytologic analysis will often yield a cell population suggestive of neutrophilic or suppurative rhinitis, indicating that chronic changes are not easily detected via cytology.8 Even with biopsy and histology, incorrect diagnosis may occur as a result of the lesion being missed. Hence in an elderly cat with rapidly progressive clinical signs, repeat biopsy is indicated if a report of rhinitis is received, as neoplasia could still be present. Sampling of nasal discharge is only of use where there is suspicion of infection with a Cryptococcus spp., as cryptococci can be easily recognized in the discharge as a round organism with a lucent halo on Romanowsky stains.9 Culture of nasal samples is of use in neutrophilic suppurative inflammation as antibiotic therapy can be tailored accurately to the infection present. In other conditions, a positive culture result is often obtained with the usual infective agents including Escherichia coli, Pseudomonas spp., Streptococcus spp., Staphylococcus spp., Pasteurella spp., Serratia spp., Klebsiella spp., and Proteus spp.7 Approximately half the cases will have mixed infections. These bacteria, however, are usually secondary invaders and whilst antibiotic treatment may temporarily improve the nasal discharge, it is unlikely to resolve the clinical signs in the long term. Nasal and paranasal sinus tumors constitute about 5% of tumors seen in the cat and are usually malignant.10 They generally originate from epithelium (with a split of 35% adenocarcinoma, 35% squamous cell carcinoma, 20% adenomas and 10% from submucosal or minor salivary gland origin) or are malignant lymphoma (30% of all nasal tumors). B-cell lymphoma is more common than T-cell.11 Other rarer tumors encountered in the nasal cavity include soft tissue sarcomas, chondrosarcoma, osteosarcoma, plasmacytoma, basal cell carcinoma, melanoma, and mastocytoma.11 Common clinical signs include nasal discharge, dyspnea, facial swelling, and epistaxis and these signs are not specific for neoplasia (Fig. 54-3). Seizures can be seen with olfactory neuroblastomas due to extension of the tumor into the brain.11 Cats with carcinomas tend to present at an older age than cats with non-epithelial tumors (12.8 years versus 8.8 years), and males are more frequently affected than female, with a 60 : 40 ratio.11 Routine staging should be performed to check for lymph node metastasis (though this is rare) or generalized disease, particularly affecting the kidney, in cats with nasal lymphoma. The majority of tumors affecting the nasal planum are squamous cell carcinoma, although other rarer tumors, including basal cell carcinoma or mast cell tumor, can occur on the planum (Fig. 54-4). Fungal granulomas do occur over the maxillary bone but tend not to originate from the actual planum. Squamous cell carcinomas are associated with exposure to ultraviolet light and thus pale sparse hair cover may predispose a cat to this disease. As multiple sites can be affected, any cats with suspicious nasal lesions should have the other predilection sites inspected, such as the pinnae and eyelids. The typical history is one of a ‘scratch’ or superficial crusting lesion that fails to heal in an older cat. The tumors have often been present for weeks/months before veterinary advice is sought. In cats, the lesion usually originates from the external cornified part of the nasal planum (Fig. 54-5). Initial staging for nasal planum tumors consists of radiography of the skull and inflated thorax. For more extensive lesions, MRI or computed tomography (CT) are useful for surgical planning. Aspirates are taken of the local submandibular lymph nodes although usually the metastatic rate is low. Figure 54-4 A six-year-old male neutered domestic short-haired cat with a soft tissue sarcoma originating from the right dorsal nasal planum. Figure 54-5 An early squamous cell carcinoma of the nasal planum in a 12-year-old male neutered domestic short-haired cat. Superficial lesions (grade Tis and small T1a, see Table 54-1) can be treated with photodynamic therapy, strontium 90 brachytherapy, radiotherapy, hyperthermia, or cryosurgery.12–17 More than one treatment may be required with both photodynamic therapy and strontium therapy, but the cosmetic results are relatively good12,13,15 (Fig. 54-6). Deeper lesions over 5 mm are likely to recur with the more conservative therapies and surgical treatment in the form of nasal planum resection is advised. Table 54-1 World Health Organization staging of feline nasal squamous cell carcinoma Nasal mesenchymal hamartomas are also known as inflammatory polyps but are distinct from nasopharyngeal polyps that originate from the bulla or Eustachian tube. Nasal mesenchymal hamartomas are firm or cystic as opposed to the firm pink masses that form nasopharyngeal polyps. Macroscopically, nasal mesenchymal hamartomas are formed of excessive mesenchymal tissue, mainly fibrous connective tissue and woven bone that is covered by ciliated columnar epithelium.18 Hence the terminology nasal mesenchymal hamartoma is more appropriate than inflammatory polyp as the lesion is disorganized indigenous tissue. Nasal mesenchymal hamartomas present in young cats, usually less than one year in age. Clinical signs include epistaxis, sneezing, stertorous breathing, and mild facial deformity while nasal discharge is unusual. Occasionally, polypoid tissue is seen protruding through the nares.19 Although these lesions are benign they can be locally aggressive with radiographic features of loss of turbinates and soft tissue opacity within the nasal cavities (Fig. 54-7). Treatment consists of surgical excision, either via a rhinotomy (see below) or per-endoscopic removal. In one case series of five cats, three were treated endoscopically, of which one had recurrence but was cured by a second treatment. One cat had spontaneous resolution of clinical signs without treatment.18 Figure 54-7 (A) An intra-oral dorsoventral view of the nasal cavity in a three-year-old male neutered cat demonstrating an expansile soft tissue opacity in the right nasal cavity with deviation of the vomer to the left. (B) A lateral skull radiograph shows increased opacity of the nasal cavity. The diagnosis in this case was nasal hamartoma (polyp). Fungal infection in the nasal cavities of cats is rare, though over the last 20 years several case reports can be found in the literature.20–26 Pre-existing nasal damage either due to a foreign body, lymphoplasmacytic rhinitis, feline rhinotracheitis virus or calicivirus, or neoplasia may compromise nasal defense mechanisms and allow fungal infections to take hold.20,22 Clinical signs usually consist of a mucopurulent chronic nasal discharge with epistaxis, sneezing, stertor, and mandibular lymphadenopathy. In cats with cryptococcocis nasal deformity may be seen. The fungal species most commonly identified are Cryptococcus spp. and Aspergillus spp., though Penicillium infection also occurs.27 Disseminated aspergillosis is comparatively common and usually occurs in immunosuppressed cats28 though FeLV/FIV infection has not been documented for the nasal cases. Cryptococcus affects males and females equally, with an over-representation of younger cats (two to three years) and Siamese, Himalayan and Ragdoll breeds.29 The nasal cavity is the most commonly affected site, with other predilection areas including the skin, especially over the nasal planum where ulcerated lesions may be seen, lungs, and lymph nodes. Cryptococcocis can be diagnosed using an immunoassay for the crytococcal polysaccharide capsular antigen (CALAS), cytologic examination, histopathology, or culture. Treatment of local disease is usually successful using azole anti-fungal drugs.27 In cases of aspergillosis, brachycephalic Persians and Himalayans are the most common breeds reported. This is the opposite of the canine cases that tend to be dolichocephalic breeds. There is no typical age group that is affected. Orbital involvement occurs in about one-third of cases with signs including exophthalmos, epiphora, anterior uveitis, resistance to retropulsion, and prolapse of the nictitating membrane.24 Diagnosis is by imaging, rhinoscopy, and biopsy samples that are submitted for culture and histopathology (Fig. 54-8). Failure to culture fungal infections other than Cryptococcus spp. is common and histology reports of fungal hyphae, rhinoscopic findings of white-gray discharge/plaques or white-yellow fungal granulomas and turbinate destruction on imaging is diagnostic. Positive aspergillosis serology on ELISA is supportive of the diagnosis though false negative results occur.20 Treatment protocols for aspergillosis include intranasal infusion of clotrimazole21 or systemic anti-fungal agents such as itraconazole, fluconazole, and ketaconazole. There are too few cases in the literature to give an accurate prognosis on feline nasal aspergillosis though the cases treated with intranasal clotrimazole seemed to have resolution of the infection in about 50% of cases. Orbital involvement appears to be a negative prognostic indicator.22,30 The skin overlying the nasal bones is a predilection site for fungal granulomas along with, to a lesser extent, other extremity sites such as digits and pinnae. These occur in cats of all ages though the incidence is more common in the middle-aged and elderly male cat.31,32 There is usually a history of a chronic non-painful subcutaneous swelling, plaque, or non-healing wound that is non-responsive to antibiotic treatment (Fig. 54-8). The usual infective agents are Alternaria spp. though others are reported including Mucor spp., Exophila spp., Ulocladium spp.31,33–35 Diagnosis involves cytologic analysis or biopsy of the swellings with histologic evidence of fungal hyphae along with fungal culture. In the case of alternariosis, the hyphae are hyaline and non-pigmented. Treatment of alternariosis is most successful with surgical debridement (Fig. 54-9) followed by anti-fungal medication, but it can also be treated with protracted medication.32 Recurrence is common. Successful treatment of the Mucor spp. case report involved five months of the second generation triazole, posaconazole.33
Nose
Surgical anatomy
General considerations
Clinical presentation
Diagnostic approach
Diagnostic imaging
Rhinoscopy
Cytology, histology and culture
Surgical diseases
Nasal and paranasal sinus tumors
Nasal planum tumors


Grade
Description
Tis
Carcinoma in situ
Superficial ulcerative/crusting lesion not extending through the basement membrane
T1a
Larger lesion <1.5 cm, exophytic
T1b
Larger lesion >1.5 cm, superficial or minimally invasive
T2
Ulcerative or infiltrative lesion of 2 cm or larger
T3
Larger lesion 2–5 cm or with invasion of subcutis
T4
Invading muscle, bone or fascia
Nasal mesenchymal hamartoma/inflammatory polyp
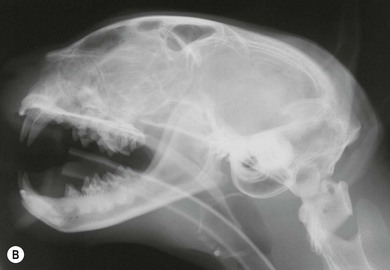
Fungal rhinitis
Fungal infections of the subcutis of the nose
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nose