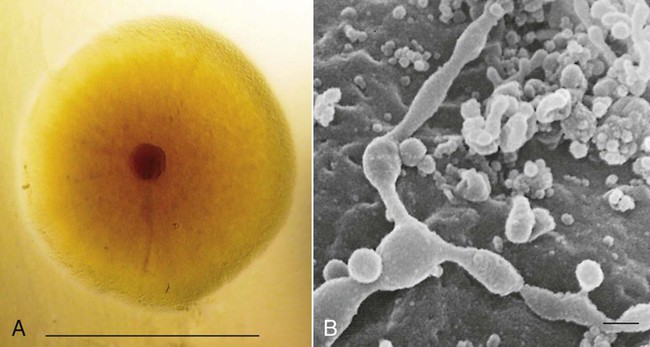
Mycoplasmas, the smallest free-living microorganisms, are prokaryotes within the class Mollicutes. Mycoplasmas are currently divided into hemotropic and nonhemotropic types. Only nonhemotropic Mollicutes are described here; hemotropic organisms are discussed in Chapter 31, Hemotropic Mycoplasmosis. Mycoplasmas have incredibly small genomes (from 606 to 1650 kb for canine mycoplasmas16), with replicating cells as small as 300 nm and a DNA molecule expressing as few as 480 genes (Fig. 32-1). Although this genome size is large enough for an extracellular existence, it restricts metabolic capacity. Mycoplasmas therefore depend on nourishment from a rich environment, which they find on mucosal membranes of the respiratory and urogenital tracts of their hosts. The lack of a rigid, protective cell wall makes mycoplasmas fragile outside the host but confers resistance to lysozyme and cell wall–inhibiting antibacterials such as penicillin, cephalosporin, vancomycin, and bacitracin. Animal mycoplasmas survive for variable time periods outside the host, depending on the species, amount of moisture, and temperature.78 Under dry conditions, most mycoplasmas are stable at 4° C, but many are unstable at higher temperatures. Stability is influenced by pH, being more stable at 7.5 than 6.5. The presence of organic material such as host tissues or body fluids helps mycoplasmal preservation. Survival times vary between species, but some can survive for 50 to 150 days at 4° C in liquid media and from 7 to 14 days under dry conditions at 30° C.53 In liquid media, that is, frozen (even at −70° C), mycoplasmal stability was unpredictable, with a marked loss of viable organisms during a period of 10 or more years. In liquid media, ureaplasmas survive for less than 14 days at 4° C. However, when lyophilized, mycoplasmas and ureaplasmas survive for 10 years or more when stored at room temperatures (22° C), 4° C, or −70° C.33 Despite their limited genome, mycoplasmas have marked genetic alterability, which allows them to vary the expression of major surface antigenic proteins as a means of avoiding host immune surveillance. Genetic studies of mycoplasmas of animals and people have shown that host preference does not correspond to bacterial phylogenetic closeness,9 suggesting that switching to a given host may have occurred at several times during mycoplasmal evolution. Nonhemotropic mycoplasmas of the genera Mycoplasma, Ureaplasma, and Acholeplasma are represented in the natural mucosal flora of dogs and cats (Table 32-1). The term mycoplasma in this chapter refers to any of these nonhemotropic organisms. Ureaplasmas require urea for fermentation and therefore have an adaptive preference for the genitourinary mucosae. Limited numbers of Ureaplasma species have been described and some have been isolated from animals and humans concurrent with disease processes, but few have been conclusively proven to be pathogenic. Table 32-1 lists diseases of dogs and cats in which mycoplasmas may be causal factors. These diseases most often involve inflammation of mucosal or serosal surfaces of the respiratory tract, urogenital tract, joints, mammary glands, and conjunctiva. Systemic spread often involves immunosuppression; however, as a result of bites or scratches, direct inoculation of mucosal organisms into deeper tissues can occur in otherwise immunocompetent animals. TABLE 32-1 Species of Mycoplasma, Acholeplasma, and Ureaplasma Isolated from Dogs and Cats NR, Not reported. aSome of these organisms were identified in clinical specimens by polymerase chain reaction directly, and not by isolation.41a,44a Studies in experimental mycoplasmal infections of mice have shown that IgA response is a primary factor in resistance to respiratory tract infections.44 Generation of this response was best induced by intrarespiratory, but not parenteral, administration of Mycoplasma antigen. Addition of cholera toxin as an adjuvant was beneficial in producing a protective immunity. Mycoplasma may also induce T-cell responses, which cause both beneficial and adverse immunologic consequences. Increased cytokines promote antimicrobial clearance and increased tissue injury. Mycoplasmal products may act as superantigens, which may stimulate chronic immune-mediated diseases such as rheumatoid arthritis. Sialidase, a bacterial virulence factor, has been found in certain species of Mycoplasma infecting dogs.71 In addition, some mycoplasmas may become intracellular, resulting in chronic persistent infections. Mycoplasmal DNA has been identified by polymerase chain reaction (PCR) methods in live virus veterinary vaccines; however, no viable organisms could be cultivated.58 Presumably, contamination during their production was the cause. Mycoplasma are common contaminants of cell culture systems in many laboratories. Mycoplasma felis is considered to be a significant pathogen in conjunctivitis of cats. For example, in cats with ocular disease from two commercial laboratories in Canada, the rates of PCR identification in corneal or conjunctival swabs of Mycoplasma spp. (11% and 27%) exceeded those of feline herpesvirus-1 (4% and 21%) and Chlamydophila felis (2% and 17%).96a Experimental inoculation of Mycoplasma has produced conjunctivitis only when cats are young or when a large amount of inoculum is used. Spontaneously observed conjunctivitis usually occurs when infected cats are housed in groups and develops soon after weaning with loss of maternal immunity. The prevalence rate of M. felis, as detected by PCR or cultivation, in conjunctival swabs from cats with conjunctivitis was 9.6% or 25%, respectively, higher than the respective rates of 2.3% or 0% found in corresponding clinically healthy cats.39,68 Results of this type from cats with and without feline conjunctivitis are compatible with the potential role of this agent. The clinical signs of mycoplasmal conjunctivitis have been described as serous discharge followed by mucoid and sticky exudate. The conjunctiva is initially hyperemic and edematous and later becomes indurated. Untreated cats may show signs as long as 60 days, but the cornea is usually not involved. However, M. felis or Mycoplasma gateae was isolated from cats with stromal ulcerative keratitis or keratomalacia, or both. Although Mycoplasma were not thought to be primary pathogens, the ocular lesions improved only after antimicrobials specific for Mycoplasma were used.35 Mycoplasma have not been associated with feline corneal sequestrate.25 Commercial PCR tests are available in a few limited laboratories for M. felis detection in cats. Mycoplasma spp. form part of the normal flora of the upper respiratory tract. However, in studies of group-living (household) cats with upper respiratory tract disease, M. felis was identified by PCR in conjunctival or oropharyngeal swab specimens of 10% of cats with clinical illness and 15% of clinically healthy contact cats.44a In contrast negative results were found in all cats from control households where clinical disease was absent. They have also been isolated at necropsy from lungs of dogs and cats with pneumonia, are not generally present in the lungs of healthy cats, but can be isolated from the lungs of healthy dogs.19,82–85 Isolation rates from dogs vary depending on the mycoplasma species in question; for example M. canis can be isolated from the lungs of 13% of healthy dogs and 24% of those with respiratory disease.19 In other studies,85 where multiple Mycoplasma spp. were examined, the total isolation rate from lungs of healthy dogs was 25% and that of ill dogs was 21%, indicating no significant difference. Ureaplasmas have been isolated from up to 13% of healthy dogs and 15% of those with respiratory disease and from dogs with pneumonia.3,19 They are rarely, if ever, isolated from the respiratory tracts of healthy or diseased cats.84 In animals with impaired pulmonary clearance resulting from viral or bacterial infection, mycoplasmas may transcend the respiratory tract and establish an infection in the lung or pleural cavity, or both, as a secondary opportunistic pathogen.26,77 Because isolation of Mycoplasma from the respiratory tract washings of clinically healthy cats is uncommon, culturing these organisms may have clinical significance. Veir et al detected mycoplasma in 80% of pharyngeal and nasal swabs from cats with respiratory disease.108 Experimental challenge with M. felis induces pneumonia in kittens, and it is likely to be a primary pathogen of cats. A kitten with congenital mitral valve dysplasia developed pneumonia and pyothorax associated with M. felis infection.69 Mycoplasma spp. were isolated in pure culture from bronchoalveolar lavage specimens from three cats with either suppurative bronchitis or bronchopneumonia.30 A Mycoplasma species was isolated from a cat with severe respiratory failure and bronchopneumonia.105 Radiographic findings were interstitial to alveolar pulmonary densities with pleural effusion. Computerized tomographic findings of the thorax included diffuse bilateral interstitial pulmonary disease, similar to findings of acute interstitial pneumonia in people caused by Mycoplasma. A predominant neutrophilic exudate was observed by cytologic evaluation of endotracheal washings. Clinical recovery occurred after treatment with doxycycline. Mycoplasma spp. and Arcanobacterium (Corynebacterium) pyogenes were isolated from pyothorax in a kitten.37 Cats with pleural effusion show minimal signs of dyspnea compared with dogs. Nonodorous fluid is expected with uncomplicated Mycoplasma pyothorax. Chronic pulmonary mycoplasmosis has been observed in dogs with primary ciliary dyskinesia.5 These bacteria have the tendency to produce prolonged suppurative infection of the conducting airways.5 Histopathologic features consist of purulent bronchitis and bronchiolitis with bronchiectasis initially, followed by bronchial and bronchiolar epithelial hyperplasia, mononuclear infiltration, lymphoid hyperplasia, interstitial pneumonia, and bronchiolitis obliterans.57 Experimental infection with M. canis, M. gateae, and Mycoplasma spumans failed to reproduce respiratory disease in dogs, whereas infection with M. cynos has been documented by infection and exposure.86,89,89 M. cynos undoubtedly contributes to the multifactorial cause of canine pneumonia, and dogs acquire infection during the first 14 days of being kenneled.19 M. cynos is found at a lower prevalence in older dogs,19,85 and antibody seroconversion to M. cynos was correlated to the development of clinical respiratory disease in dogs entering a kennel.96 Pulsed-field gel electrophoresis and random amplification of polymorphic DNA analysis has indicated that kenneled dogs can be infected with identical clonal types of M. cynos and that specific types can persist in kenneled populations.70 Mycoplasmas have been isolated, alone and in combination with other bacteria or viruses, from the airway washings of dogs with lower respiratory diseases.20,22 Common but variable anatomic changes in these dogs included collapsing trachea and interstitial, bronchointerstitial, and alveolar pulmonary disease. Small (0.3 to 0.9 µm) pleomorphic coccoid organisms, characteristic of Mycoplasma, were found in large numbers within neutrophils of transtracheal wash fluid from a 4-month-old pup with bronchointerstitial pneumonia from which Mycoplasma sp. was cultured.110 Escherichia coli was also cultured from this dog; however, it was considered to be a contaminant. Additional convincing evidence of the primary pathogenic role of M. cynos was its isolation from a litter of 3-week-old pups with severe respiratory disease.112 Concurrent viral infections were not confirmed by PCR of conjunctival swabs and whole blood. Clinical findings were fever (at or above 41° C [105.8° F]), productive cough, and leukocytosis with a left shift. Radiographic abnormalities were alveolar and bronchointerstitial pulmonary densities, mild pleural effusion, and enlarged mediastinal lymph nodes. Some of the pups died despite treatment with amoxicillin, which does not have efficacy against cell-wall free organisms such as Mycoplasma. Necropsy findings were acute generalized mucopurulent airway exudates with partly hemorrhagic fibrinous necrotizing bronchopneumonia. M. cynos was found in neutrophilic inflammatory sites of the lung by immunohistochemical staining. M. cynos was isolated in pure culture in large numbers from pulmonary tissue. Organisms and lesions were found only in the respiratory tissues of the dead pups. Both M. cynos and M. canis were isolated from pharyngeal swabs of the surviving pups. Lincomycin was administered to the surviving pups, which showed minimal improvement. Dramatic and complete recovery was observed after treatment was switched to erythromycin. Isolating mycoplasmas in large numbers from the urine of dogs with urinary tract infection is not uncommon.93 Although in most situations the mycoplasmas are in mixed culture with bacteria, they are also occasionally isolated in pure culture.48,62,62 The same conditions, such as tumors and urinary calculi, that predispose the animal to bacterial infection may promote Mycoplasma infection. The source of the mycoplasmas in the urinary tract is undoubtedly the abundant microflora in the distal urogenital tract.60,61 More credibility exists for their role in infection when urine is collected by cystocentesis or tissue by biopsy via laparotomy and no other bacteria are isolated. Collection of urine by voiding or catheterization will result in contamination of the urine specimen. Conditions causing obstruction and urine stasis of the urethra will allow ascending contamination of the urinary system so that they may be isolated with cystocentesis. M. felis and M. gateae have not been able to survive under osmotic conditions present in normal feline urine,11 indicating that mycoplasmas are unlikely candidates for urinary disorders of cats. Ureaplasma spp. are more resistant to osmotic damage by urine and are more likely candidates for urinary tract infections in dogs and cats.11 No features can be used to distinguish mycoplasmal urinary tract infection from other bacterial urinary infections. Studies in cats with feline lower urinary tract disease have not implicated either mycoplasmas or ureaplasmas.1,97 Mycoplasmas of the reproductive tract are currently considered to be opportunistic. However, M. canis has been isolated from dogs with endometritis, and experimental infection induced chronic urethritis and epididymitis in 50% of males and enlarged uterus and endometritis in females.91 Further studies are needed to clarify the role of M. canis in genital infection and infertility. The assumed opportunistic role of mycoplasmas in endometritis is probably overlooked in many cases of mixed infection when conventional bacteria are cultured. Alternatively, opportunistic bacterial infections may be responsible for the reproductive disorders in kennels where both mycoplasmas and bacteria are found. Vaginal and preputial swabs and semen samples are often submitted for mycoplasmal culture in dogs with infertility problems. Very often, these samples give positive results; however, their significance as pathogens is uncertain. Weighing the culture results in relation to other diagnostic findings and judging whether antibacterial treatment for mycoplasmas will solve the infertility problem are important. Deep vaginal or semen cultures are the most accurate means of determining the presence of the organisms. A survey showed that infertile dogs had ureaplasmas in vaginal and preputial samples more often than did fertile dogs. This difference was statistically significant in male dogs but not in female dogs.27 Because ureaplasmas are associated with infertility in other animals, research work is urgently needed to evaluate the role of these organisms in dogs and cats. The percentages of dogs with positive culture results for Mycoplasma from the vagina or semen have not been statistically different between fertile and infertile dogs.28
Nonhemotropic Mycoplasmal, Ureaplasmal, and L-Form Infections
Nonhemotropic Mycoplasmal and Ureaplasmal Infections
Etiology
Situation
Dogs
Cats
Upper respiratory tract (oropharynx, nasopharynx, larynx) of clinically healthy animals
M. arginini, M. canis, M. cynos, M. edwardii, M. felis, M. feliminutum, M. gateae, M. maculosum, M. molare, M. opalescens, M. spumans, Unclassified Mycoplasma spp. HRC 689 and VJC358, Ureaplasma spp., U. canigenitalum19,85
Acholeplasma spp., M. arginini, M. arthritidis, M. feliminutum, M. felis, M. gallisepticum, M. gateae, M. pulmonis, Ureaplasma spp.84 U. cati, U. felinum40
Lower respiratory tract (lung and tracheobronchial secretions) of healthy animals
M. arginini, M. canis, M. cynos, M. edwardii, M. felis, M. maculosum, M. spumans, Ureaplasma spp., Unclassified Mycoplasma sp. HRC 68919
M. felis, M. gateae82,84
Lower respiratory tract of pneumonic animals
M. arginini, M. bovigenitalium, M. canis, M. cynos, M. edwardii, M. gateae, M. feliminutum, M. maculosum, M. spumans, Unclassified Mycoplasma spp. HRC 689 and VJC358, Mycoplasma spp., Ureaplasma spp.19,47,57,85
M. arginini, M. felis20,77
Pleuropulmonary abscesses
NR
Mycoplasma spp.37,69,69
Conjunctiva of healthy animals
M. bovigenitalium, M. canis, M. cynos, M. spumans13,16
None39,68
Conjunctivitisa
NR
M. felis,39,44a,79 Mycoplasma spp.,68 M. canadense,41a M. lipophilum, 41a M. hyopharyngis, 41a M. cynos41a
Peritoneal cavity
NR
NR
Arthritis
M. spumans4, M. edwardii101
M. gateae,76,111 M. felis67
Genital tract
A. laidlawii, M. bovigenitalium, M. canis, M. cynos, M. edwardii, M. feliminutum, M. gateae, M. maculosum, M. molare, M. opalescens, M. spumans, Ureaplasma canigenitalum, Unclassified Mycoplasma sp. HRC 689, Mycoplasma spp.62,65
M. felis, M. gateae, Ureaplasma spp.11
Urine
M. canis, M. spumans, M. cynos48,62
NR
Epididymo-orchitis
M. canis62
NR
Meningoencephalitis
M. edwardii46a
NR
Abscesses
NR
Mycoplasma-like organisms: cat bites,b M. canis, M. spumans. dog bite104
Clinical Findings
Ocular Infections
Cat
Respiratory Infections
Cat
Dog
Genitourinary Infections
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nonhemotropic Mycoplasmal, Ureaplasmal, and L-Form Infections