Chapter 1 Non-human primates
Cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) macaques and the common marmoset (Callithrix jacchus)
Cardiovascular system
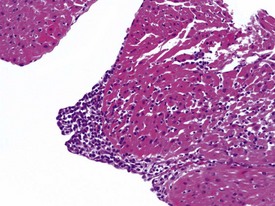
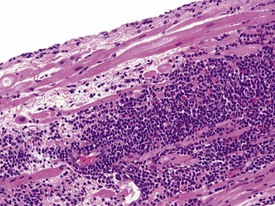
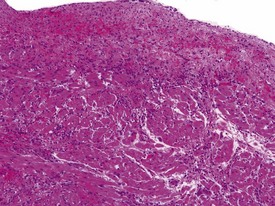
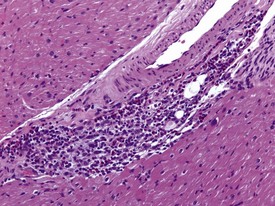
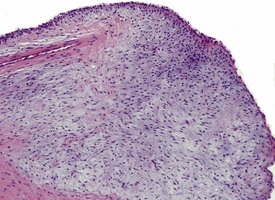
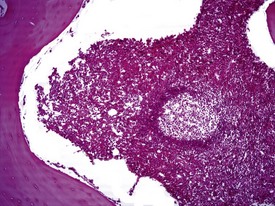
Spontaneous findings of the heart are among the most commonly encountered findings in non-human primates used as controls in preclinical safety studies. Of these, the most frequently recorded incidental findings are idiopathic myocardial inflammatory cell infiltrations and focal myocarditis. Although various diagnostic terms have been used to describe these two seemingly separate inflammatory findings of the myocardium (Drevon-Gaillot et al 2006, Keenan & Vidal 2006, Lowenstine 2003, Qureshi 1979, Scott, 1999, Shimoi et al 1998), it is generally accepted that they represent a single pathological entity that differs only in the degree of severity (Chamanza et al 2006). The pathology continuum starts with minimal to mild, focal, lymphoplasmacytic inflammatory cell infiltration of the myocardium, with little or no evidence of cardiac myocyte necrosis or degeneration (Fig. 1.1), and progresses to focal myocarditis characterized by some evidence of cardiac myocyte necrosis and associated edema or fibrin deposition (Fig. 1.2). Both conditions occur more commonly in the subendocardial or subepicardial areas of the heart and may occur simultaneously in the same heart. No clinical signs or grossly visible lesions are known to be associated with these findings, and no etiological agent has been isolated or demonstrated (Chamanza et al 2006). The most likely cause is therefore considered to be stress and the release of catecholamines associated with capture or captivity; hence, higher incidences of the findings have been reported in wild-caught animals (Qureshi 1979) compared with laboratory non-human primates. A proposal has been put forward to apply umbrella terms such as ‘focal idiopathic myocardial inflammation’ for these lesions and to grade them according to the presence or absence of degenerative or inflammatory changes in the adjacent myocardial tissues when evaluating non-human primate hearts in routine toxicity studies (Chamanza et al 2006).
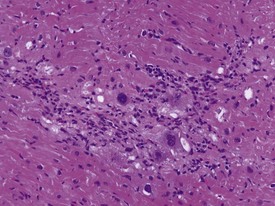
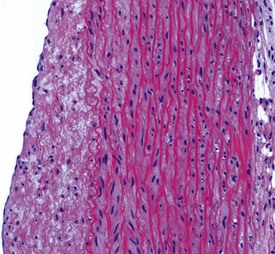
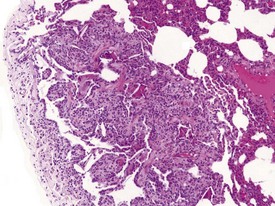
Myocardial degeneration with karyomegaly is an idiopathic focal myofibre degeneration or cardiomyopathy (Vidal et al 2010, Zabka et al 2009) that has been described in cynomolgus macaques from various sources and origins. Even though it has occurred at a low frequency, high incidences have been recorded in the few studies in which it has occurred (Chamanza et al 2010). The finding is characterized by minimal to moderate focal myocardial degeneration associated with mild to moderate karyomegaly, cardiac myofibre hypertrophy and vacuolation, with minimal inflammation or fibrosis (Fig. 1.3). In early lesions, only lipid-depleted vacuolation of cardiac myofibres and karyomegaly with increased nuclear basophilia, are present, while more advanced lesions may be associated with inflammation, hemorrhage, mineralization and extensive fibrosis. The most commonly affected areas of the heart are, in decreasing order, the subepicardial areas of the apex, the interventricular septum (just below the atrio-ventricular valves), and the tip of the papillary muscle and subendocardial areas of both ventricles (Chamanza et al 2010).
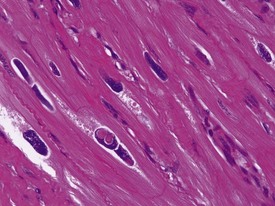
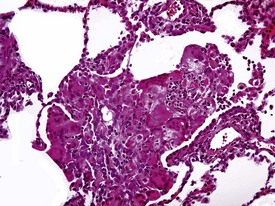
Rarely eosinophilic intranuclear inclusion bodies, believed to be caused by invagination of the cytoplasm into the enlarged nuclei, and intracytoplasmic eosinophilic or hyaline granules (usually located at the poles of the nucleus) within the hypertrophic cardiac myofibres, may be observed (Fig. 1.4). Intranuclear (pseudo)inclusion bodies in hypertrophic cardiac myofibres resulting from intranuclear invagination of cytoplasmic organelles, including mitochondria, have been reported in man (Engedal et al 1977), while hyaline, ceroid and lipofuscin cytoplasmic granules located at the poles of cardiac myofibre nuclei have been reported in cynomolgus monkeys (Jasty et al 1984).
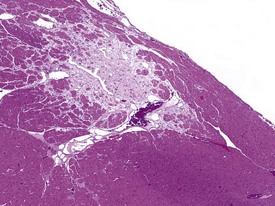
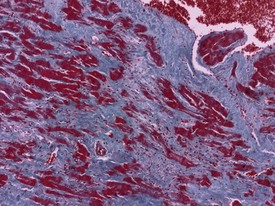
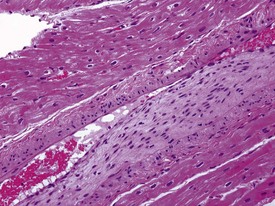
Acute hemorrhagic necrosis (Fig. 1.5) and/or fibrosis (Fig. 1.6a & b) of the papillary muscle or subendocardial areas, resembling ischemic lesions such as those associated with beta-agonist and other cardio-active drugs (Greaves 2000), have also been observed in occasional animals, including marmosets (Fig. 1.6a & b).
The possible role of catecholamines in this idiopathic myocardial degeneration has been suggested. Findings similar to those described above, such as cardiac myofibre hypertrophy, karyomegaly and vacuolation, have been observed in a rhesus macaque (Macaca mulatta) with an active angiomatous phaeochromocytoma (Vogel & Fritz 2003) and in animals directly injected with catecholamines (Khullar et al 1989).
Ectopic cysts or glandular structures lined by various types of epithelium, from squamous to cuboidal, or columnar, have been described in various species, including man (De Lacroix & Hübner 1974, Thomas & van Wesep 1990), cattle (Bundza & Dukes 1978) and mice (Elwell & Mahler 1999). In non-human primates, three main types of ectopic cyst have been described in the cynomolgus and rhesus macaques and marmosets (Chamanza et al 2006, Drevon-Gaillot et al 2006, Kaspareit et al 2003).
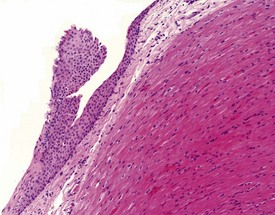
Keratinized or non-keratinized squamous cysts, squamous plaques with no central lumen (Fig. 1.7), and thyroid follicle-like epithelial cysts filled with an eosinophilic fluid have been reported at very low incidences in the three species. Squamous keratinized cysts are lined with a complete or incomplete thin layer of flattened epithelium filled with concentric layers of keratin (Fig. 1.8) and may be associated with a foreign-body inflammatory reaction where the epithelial wall has been broken, or appears incomplete, and the keratin is in direct contact with adjacent tissues (Chamanza et al 2006). Squamous non-keratinized cysts are composed of a thicker layer of stratified squamous epithelium around a central lumen filled with a few inflammatory cells, amorphous cellular debris or eosinophilic colloid-like material. The base of the epithelial wall of the non-keratinized squamous cyst is usually surrounded by a layer of fibrous connective tissue of varying thickness.
Squamous plaques, which are incomplete cysts with no central lumen, may be found lying adjacent to squamous cysts, in which case they are thought to represent tangential sections of walls of adjacent squamous cysts. However, some epicardial or endocardial squamous plaques, unassociated with complete squamous cysts, have also been observed (Fig. 1.7). Keratinized or non-keratinized squamous cysts and squamous plaques are usually located in the subepicardial or subendocardial regions between the apex and the base of the heart, and are therefore easily identified as yellow-white nodules on the heart surface or interventricular septum at necropsy.
Ectopic thyroid tissue or thyroid follicle-like structures have been observed mainly in the subepicardial areas at the base of the heart, including atrial appendages and walls of the great vessels, and are not readily identified at necropsy. Squamous cysts and squamous plaques may be of foregut origin while ectopic thyroid tissue could be of thyroglossal duct origin (Elwell & Mahler 1999, Kaspareit et al 2003).
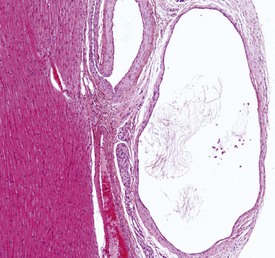
Various types of vascular lesion have been reported to occur spontaneously, albeit at very low frequencies in laboratory non-human primates (Chamanza et al 2006, 2010). With the exception of continuous intravenous infusion studies, in which local injection sites and systemic vascular lesions are common background findings associated with the test item administration procedure (Lilbert & Burnett 2003), inflammatory lesions of blood vessels are uncommon in laboratory non-human primates.
Two isolated cases of polyarteritis nodosa, a systemic necrotizing vasculitis of small to middle-sized arteries, have been described in cynomolgus macaques (Albassam et al 1993, Porter et al 2003). The most frequently encountered inflammatory vascular lesions are minimal to moderate, localized vasculitis or perivasculitis characterized by a lymphoplasmacytic infiltration of the vascular wall without extensive necrosis of the tunica media or substantial fibrin deposition. The most commonly affected organs include the large intestinal wall, lungs, meninges (brain and spinal cord), heart (Fig. 1.9), urinary bladder and sciatic nerve.
Naturally occurring, degenerative lesions of arteries, such as atherosclerosis, do not occur commonly in non-human primates, but intimal thickening and the formation of arteriosclerotic lesions (fibrous plaques) in the coronary artery and aorta, sometimes leading to occlusion, are encountered infrequently (Chamanza et al 2010, Scott 1999). The intima is infiltrated by smooth muscle cells, mucopolysaccharides and fibrous tissue with little or no foam cells or extracellular lipids seen in diet-induced, atherosclerotic plaques (Fig. 1.10). Intimal proliferation or fibrocellular intimal thickening may also be encountered in the lung of normal control cynomolgus macaques following organization of small pulmonary emboli derived from infusion sites.
Another closely-related, degenerative lesion of arteries seen in young macaques is the accumulation of mucopolysaccharides, or mucification, in the subendothelial areas of the aorta and the heart. Mucin accumulation in the tunica intima and, to a lesser extent, tunica media, of the aorta, with proliferation of intimal cells and splitting of elastic fibres of the tunica media (Fig. 1.11a), has been observed at low frequency in cynomolgus macaques used in toxicity studies (Chamanza et al 2010). Mucin or mucopolysaccharide accumulation in the tunica intima of the aorta and subendocardial tissues of the heart chambers and valves (Fig. 1.11b) has also been described in humans and other non-human primates (Lindsay & Chaikoff 1966, Scott 1999). The mucopolysaccharide content of the walls (particularly the intima) of arteries of non-human primates is generally more abundant than it is in humans (Scott 1999). This aortic degenerative change in macaques results in a massive accumulation of mucopolysaccharides in the subintimal areas, causing localized swelling or expansion of the intima with disruption of the elastic fibres of the intima media (Fig. 1.11a).
Hemolymphoreticular system
Extramedullary hematopoiesis (EMH) occurs frequently in the adrenal gland (Fig. 1.53), liver, kidney and other tissues in marmosets. The severity and incidence of this finding is usually associated with the frequency of blood sampling, with marmosets being very sensitive to frequent bleeds (Tucker 1984). This spontaneous finding should be differentiated from inflammation, inflammatory-cell infiltrates or treatment related EMH. Spontaneous EMH in macaques is extremely rare, but has sporadically been observed in lymph nodes of healthy control cynomolgus macaques (Chamanza et al 2010).
Multinucleate lymphocytic syncytia, resembling the Warthin–Finkeldey bodies associated with measles (Fig. 1.12), have been observed with some frequency in the bronchial-associated lymphoid tissue (BALT) of the larynx and the gut-associated lymphoid tissue (GALT) of the large intestines in normal healthy cynomolgus macaques (Chamanza et al 2010). It is not known whether these represent subclinical measles infection, but no necrosis of lymphocytes is observed within the lymphoid nodules, nor are viral inclusions present within the cells. Natural measles infections which may originate from animal handlers, are known to occur in both marmosets and macaques (Scott 1999).
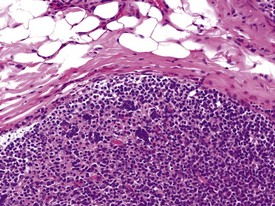
FIGURE 1.12 Warthin–Finkeldey bodies in the gastrointestinal lymphoid tissue (GALT) of a non-human primate. ×100.
The presence of prominent lymphoid follicles within the bone marrow is a common background finding in macaques (Fig. 1.13). This lesion, together with lymphoid follicular hyperplasia and prominent germinal centres in other sites, such as the spleen (Fig. 1.14), lymph nodes and salivary glands, have been associated with subclinical type D retroviral infection (Guzman et al 1999, Lowenstine 1993). However, most non-human primates used in preclinical studies are routinely screened for, and are known to be free from, simian retroviruses (type D). Thus hyperplastic lymphoid follicles in the spleen and other tissues are considered to indicate heightened non-specific immunosurveillance in animals which have been reared in a relatively disease-free environment and are known to be free from major pathogens. Periodontal disease, glossitis and tonsillitis are other common minor inflammatory lesions known to be associated with lymphoid follicular hyperplasia and mononuclear cell infiltrations in other organs.
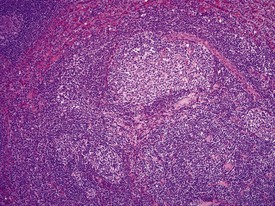
FIGURE 1.14 Prominent lymph follicle with hyalinization in the lymph node of a non-human primate. ×100.
The accumulation of brightly eosinophilic amorphous material (hyalinization) in the centre of lymphoid follicles in non-human primate spleens is a common observation of no known clinical significance. The eosinophilic substance is thought to be proteinaceous in content and composed of antigen–antibody complexes. Russell bodies can occasionally be observed in the vicinity of hyalinized germinal centres (Fig. 1.14), giving more credibility to the theory of immunoglobin composition of the amorphous material.
Respiratory system
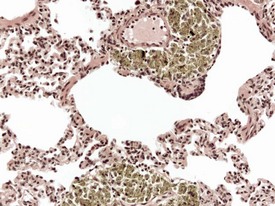
Pneumonia in laboratory macaques is not common, but focal interstitial inflammation or fibrosis of minimal to slight grades of severity, does occur with some frequency. The lesions occur in subpleural areas or at the tips of lung lobes. There may be proliferation of bronchiolo-alveolar epithelium, fibrosis and accumulation of alveolar macrophages in alveolar spaces (Fig. 1.15). Other associated lesions commonly encountered in the lungs of macaques include focal pleuritis and pleural or subpleural fibrosis, with or without lung adhesions to adjacent lobes or to the parietal pleura. These are usually associated with necropsy findings of lung adhesions or pale white foci of the pleura.
Other inflammatory lesions of the lung include focal, foreign-body granulomas with minimal to mild interstitial inflammation. The lesions occur as a result of inhalation of plant, food or other small particles (Fig. 1.16) and should be distinguished from hair emboli, which usually occur as a result of the inadvertent injection of hair or skin fragments during intravenous injection of test substances. Hair emboli or fragments of skin lodged in macaque lungs may appear as focal, foreign-body granulomas in the lung or within arterial thrombi with a perivascular reaction (Kast 1994).
Pulmonary vascular disease in laboratory primates is rare, but arteritis/periarteritis, thromboembolism, perivascular edema and arteriosclerosis may be encountered in control animals. Focal thickening of the intima, due to cellular proliferation in earlier lesions and later replacement by fibrosis (with or without smooth muscle hyperplasia) of the media can be encountered. Intimal lesions can lead to complete occlusion of the artery concerned (Fig. 1.17), but changes in adjacent tissues are usually absent. However, occlusive intimal proliferation and thrombosis associated with continuous intravenous infusion with saline may be associated with bronchopneumonia, which is usually confined to the distal parts of lung lobes, due to the dual blood supply of the lungs. Diffuse interstitial inflammation, periarteritis and perivascular edema are some of the more common findings observed in saline-injected control animals on continuous intravenous infusion studies.
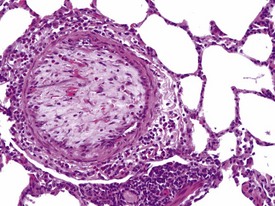
FIGURE 1.17 Thrombi and intimal proliferation in a blood vessel of the lung of a non-human primate. ×200.
Yellowish brown to dark brown pigment in alveolar macrophages is often found distributed perivascularly or peribronchiolarly within the lungs of macaques (Fig. 1.18). Although iron-positive pigment associated with the lung mite Pneumonyssus simincola is occasionally encountered, especially in wild-caught macaques, the majority of the brown pigment present in the lungs of purpose-bred macaques is usually not associated with any parasite sections or pulmonary parenchymal pathological changes, and is therefore believed to be anthracosis. Anthracosis, or pneumoconiosis, is caused by the inhalation of atmospheric carbon particles by laboratory non-human primates housed near or within urban areas. Similar pigment can be encountered within macrophages in the bronchial or mediastinal nodes and should be differentiated from tattoo pigment.
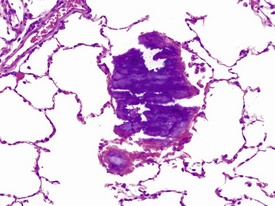
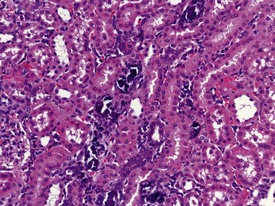
Vascular or parenchymal mineralization in the lungs (Fig. 1.19) of marmosets occurs commonly in control animals. This occurs as part of metastatic mineralization in this species and may involve other major organs such as the kidneys, the heart (mostly the atria), the aorta and adrenal glands. Excessive calcium absorption as a result of hypervitaminosis D, or high calcium levels in the diet, are believed to be the main causes of metastatic calcification in marmosets (Kaspareit 2006, Scott 1999). The kidneys and the urinary tract are the most commonly affected areas. Mineralization of other organs, such as the lungs, can also be observed in marmosets with severe renal disease and nephrocalcinosis (Fig. 1.20) which can complicate the etiological diagnosis. Metastatic calcification in macaques is very rare, and almost all forms of mineral deposition seen spontaneously are dystrophic in nature.
Gastrointestinal system
Superficial inflammation, erosion or ulceration of the tongue and esophageal mucosa (Fig. 1.21), associated with Candida albicans infection, is occasionally encountered in stressed and debilitated marmosets (Chalmers et al 1983) or macaques on long-term antibiotic treatment. The lesion is characterized grossly by the presence of pale pseudomembranes on the oral or esophageal mucous membranes, with superficial ulceration beneath the pseudomembrane. Upon histopathological examination, erosion and ulceration that rarely extend below the basement membrane are observed in association with minimal, mixed inflammatory cell infiltrates and fungi in the superficial epithelium. The organisms consist of septate pseudohyphae and budding blastospores and are easier to visualize with PAS or Grocott Gomori’s methenamine silver stains.
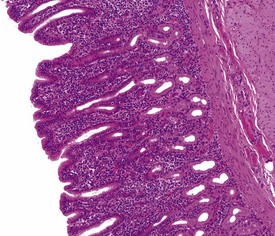
The normal histology of the stomach of the cynomolgus macaque is of particular importance because some pathological changes affecting the mucosa are associated with an increase or decrease in some cell populations that make up the gastric mucosa. The stomach of the cynomolgus macaque can be divided into four anatomical regions: the cardia, fundus, body and antrum. It is the histological appearance of the fundic region of the stomach in the non-human primate that differs slightly from that of humans and dogs. Unlike humans, dogs and pigs, the fundic region and the body of the stomach of macaques are histologically different, with the fundic mucosa having few or no parietal cells and the transition between the two zones being marked by the abrupt appearance of parietal cells (Vidal et al 2008). The histological appearance of the antral mucosa does not differ greatly from that of humans and dogs and has numerous, deep gastric pits, no parietal cells and a few chief cells. Lymphoid follicles, or foci of lymphoplasmacytic cells, can be observed throughout the gastric mucosa in normal stomach sections, but these can be increased in gastritis.
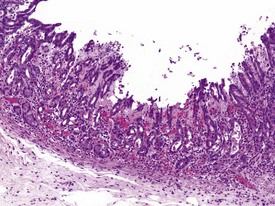
Gastritis, especially chronic gastritis of the antral region, is very common in laboratory non-human primates and can affect the majority of animals in a toxicity study (McInnes & McKeag 2011). Chronic gastritis is characterized by minimal to marked infiltration of the mucosa and submucosa with lymphocytes and plasma cells, an increase in lymphoid follicles and mucosal intestinalization of the antral mucosa typified by atrophy of gastric glands with an accompanying increase in the lamina propria area (Fig. 1.22). While chronic gastritis is observed mainly in the antral mucosa, acute or chronic-active gastritis associated with hemorrhage, erosions or glandular micro-abscesses is more common in the fundic/body of the stomach (Fig. 1.23).
Helicobacter pylori and Helicobacter heilmannii-like organisms (also known as Gastrospirillum hominus) are the most commonly identified pathogens in the stomach of non-human primates used in toxicity studies and are believed to be associated with subclinical gastritis (Dubois et al 1994, Reindel et al 1999). Helicobacter pylori is a small (2–4 µm long) bacterium, shaped like a comma, a loose spiral or a seagull’s wing, that occurs largely in the gastric antral mucosa. Lesions associated with H. pylori include a diffuse, mononuclear cell infiltration of the mucosal lamina propria with mucosal thickening, lamina propria expansion with separation of glands, epithelial hyperplasia, prominent lymphoid follicles or mucosal erosion. The lymphoplasmacytic infiltrates can be made up of a large percentage of plasma cells with Russell bodies but occasionally, and in the more severe lesions, neutrophilic infiltrates in the glandular lumen (glandular micro-abscesses) may be observed.
Helicobacter heilmannii-like organisms (HHLOs) are larger than H. pylori (approximately 7–10 µm) and have a tightly wound spiral shape with approximately 4 to 12 coils. They typically colonize the fundic/body mucosa and can be observed within parietal cells. Although HHLOs are more prevalent than H. pylori in the stomach of both cynomolgus and rhesus monkeys, evidence shows that HHLOs are relatively non-pathogenic, although subtle damage to infected parietal cells has been reported (Dubois et al 1991).
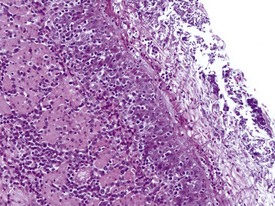
Gastric infarction is an uncommon, but clinically important, finding in young adult cynomolgus macaques. It may be the cause of death or poor health in study animals or may be observed as an incidental finding in clinically normal animals. The cause of gastric infarction is not always apparent but in most cases a severe systemic insult that predisposes to disseminated intravascular coagulation, such as trauma, hernia, intussusceptions or some other natural disease, is usually present (Chamanza et al 2010, Fikes et al 1996). The lesion is characterized by extensive necrosis, haemorrhage and edema of the muscularis and submucosa of the fundic or pyloric region of the stomach (Fig. 1.24) associated with micro-thrombi, particularly in the venous system.
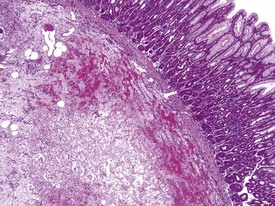
FIGURE 1.24 Extensive necrosis in the submucosa, due to microthrombi, in the stomach of a non-human primate. ×100.
The presence of pigment-laden macrophages within the lamina propria of the small intestinal villi tips is a very common finding in laboratory macaques. The finding has been reported at high incidences by several authors (Drevon-Gaillot et al 2006, Lowenstine 2003, Scott 1999), and various theories have been suggested regarding the nature of the pigment. The yellowish to light brown, granular pigment is usually associated with apoptotic bodies within the lamina propria without any associated damage to the enterocytes. The ileum is the most affected small intestinal segment (Fig. 1.25). Iron (Scott 1999), bile pigment, ceroid-lipofuscin and necrotic cellular debris (Drevon-Gaillot et al 2006, Ito et al 1992) have been suggested to be part of the constituents of this pigment. The significance of this finding has not been determined and it may represent attempts by lamina propria phagocytes to engulf foreign material.
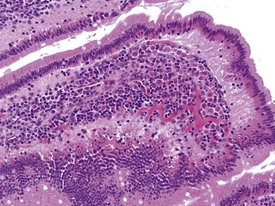
FIGURE 1.25 Pigmented macrophages in the lamina propria of the small intestine of non-human primates. ×200.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree