CHAPTER 8 Neurulation
Neurulation is a fundamental event of embryogenesis. It leads to the formation of the neural tube, the precursor of the central nervous system including the brain and spinal cord. This organ system is the first to initiate its development; functionally, however, it is overtaken by the development of the vascular system, which is the first organ system to gain function.
FORMATION OF THE NEURAL TUBE: PRIMARY AND SECONDARY NEURULATION
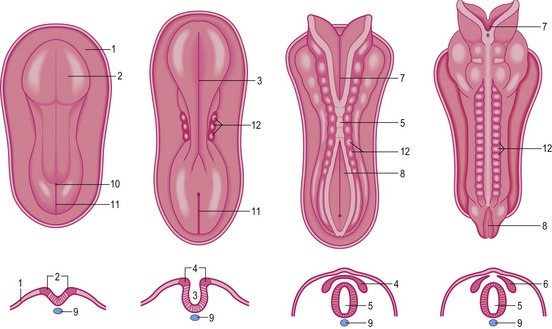
During neurulation, which is conventionally divided into primary and secondary phases, the epiblast is induced to form neural ectoderm (Fig. 8-1). The first morphological sign of primary neurulation is a dorsal thickening in the anterior ectoderm concomitant with regression of the primitive streak. This elliptical region of specialized thickened ectoderm is referred to as the neural plate. Subsequently, the neural plate undergoes a shaping which converts it into a more elongated key-hole-shaped structure with broad anterior and narrow posterior regions. The main driving force of neural plate shaping seems to come from convergent extensions that cause a net medially directed movement of cells with intercalation in the midline. This leads to lengthening and narrowing of the neural plate. Neural plate shaping is followed by the development of two lateral elevations, the neural folds, on either side of a depressed midregion referred to as the neural groove. In pigs and cattle the neural folds become clear during the third week of development. Development of the anterior neural folds seems to be highly dependent on the underlying mesenchyme which proliferates and expands markedly, with a notable increase in its extracellular spaces, as the elevation of the folds begins. In the spinal part of the neural tube, expansion of the paraxial neural fold elevation is not accompanied by elevation of mesenchyme.
Bending of the neural plate and apposition of neural folds
The bending of the neural plate, which is observed during primary neurulation, occurs at three principal sites: the median hinge point (MHP), overlying the notochord, and the paired dorsolateral hinge points (DLHP) at the points of attachment of the surface ectoderm to the outside of each neural fold. The MHP is induced by signals from the notochord and is the sole site of bending in the upper spinal neural plate.
Fusion of neural folds
Fusion begins in the cervical region and proceeds anteriorly and posteriorly from there. This fusion is mediated by cell-surface glycoconjugates. As a result of these processes, the neural tube is formed and separated from the overlying ectodermal sheet. Until fusion is complete, the anterior and posterior ends of the neural tube communicate with the amniotic cavity via two openings, the anterior and posterior neuropores. Closure of the anterior neuropore occurs in the bovine embryo at approximately Day 24 (18 to 20 somite stage), whereas the posterior neuropore closes two days later (25 somite stage). Neurulation is then complete. The central nervous system is represented at this time by a closed tubular structure with a narrow posterior portion, the anlage of the spinal cord, and a much broader cephalic portion, the primordium of the encephalon. During neurulation, the neuroepithelium is entirely proliferative; cells do not begin to exit the cell cycle and start neuronal differentiation until after the neural tube closure is complete.
NEURAL CREST
Formation of neural crest
As the neural folds elevate, cells of the lateral border or crest of the neuroepithelium, the neural crest, undergo an epithelio-mesenchymal transition as they leave the neuroectoderm by active migration into the underlying mesoderm (Fig. 8-1). Induction of neural crest cells requires interactions between neural and overlying surface ectoderm. Neural crest cells are specified as the result of an inductive instruction by the surface ectoderm. The instruction is possibly mediated by a gradient of bone morphogenetic protein 4 (BMP4), BMP7, and Wnt. Bone morphogenetic proteins secreted by surface ectoderm initiate this process. Thus stimulated, neural crest cells express slug, a transcription factor of the zinc finger family that characterizes cells that break away from an embryonic cell layer to migrate as mesenchymal cells.
Migration of neural crest cells
The pathway by which the neural crest cells leave the neural tube depends on the region. Migrating crest cells give rise to a heterogeneous array of cells and tissues. Neural crest cells that leave the anterior parts of the neural folds, before closure of the neural tube in this region, contribute to the craniofacial skeleton and other mesenchymal derivatives, but can also differentiate into several other cell types including neurons of the cranial ganglia, Schwann cells and melanocytes Table 8-1.
Table 8-1: Major derivatives of the cranial and circumpharyngeal neural crest
| Sensory nervous system | Ganglia of trigeminal nerve (V), facial nerve (VII), glossopharyngeal nerve (superior ganglion), vagus nerve (jugular ganglion) |
| Autonomic nervous system | Parasympathetic ganglia: ciliary, ethmoidal, sphenopalatine, submandibular, visceral |
| Satellite cells of sensory ganglia, Schwann cells of peripheral nerves, leptomeninges of prosencephalon and part of mesencephalon | |
| Endocrine cells | Carotid body, parafollicular cells of thyroid |
| Pigment cells | Melanocytes |
| Mesectodermal cells | Cranial vault (squamosum and part of os frontale), nasal and orbital bones, part of the otic capsule, palate, maxilla, visceral cartilage, part of external ear cartilage |
| Connective tissue | Dermis and adipocytes of the skin, cornea of the eye, odontoblasts, stroma of glands (thyroid, parathyroid, thymus, salivary, lachrymal), outflow tract of heart, cardiac semilunar valves, walls of aorta and aortic-arch derived arteries |
| Muscle | Ciliary muscle; dermal smooth muscles, vascular smooth muscle |
A significant change accompanying the epithelial-mesenchymal transition of the neural crest cells is their loss of cell adhesiveness due to loss of adhesion molecules (CAMs) characteristic of the neural tube (e.g. N-CAM, and N-cadherin).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree