CHAPTER 27 Neurology
Neurologic diseases in cats present a unique challenge to the feline practitioner because of the inherent difficulties of examining feline patients. Cats are not prone to the same diseases as dogs, and often clinical signs in cats may be atypical, further complicating the veterinarian’s ability to develop a neuroanatomic diagnosis. The neurologic exam may be markedly altered in cats as a result of their high sympathetic overdrive and their physiologic responses to stress; therefore, in many instances, unless the patient is extremely obtunded or unusually compliant, a complete examination may not be possible. Because the examiner may have difficulty even eliciting the most basic reflexes and postural responses in an apprehensive cat, minimal restraint, a quiet environment, and extreme patience are imperative. Although a complete neurologic assessment may not always be possible in a fractious cat, a thorough history, physical examination, and observation of the cat in most cases will allow the practitioner to develop a neuroanatomic diagnosis. On the basis of this diagnosis, the veterinarian can generate a reasonable list of differentials and determine appropriate ancillary testing. Detailed descriptions of individual disorders and abnormalities on the neurologic exam are discussed in the following sections. For a detailed description of the neurologic exam, the reader is referred to Veterinary Neuroanatomy and Clinical Neurology, by Dr. A. DeLahunta and Dr. R. Glass (Box 27-1).
BOX 27-1 Additional Resources
DeLahunta A, Glass R: Veterinary neuroanatomy and clinical neurology, St Louis, 2009, Saunders.
Brain tumors in dogs and cats, North Carolina State College of Veterinary Medicine: http://cvm.ncsu.edu/vth/clinical_services/neuro/brain_tumor.html
Comparative neuromuscular laboratory, University of California, San Diego: http://vetneuromuscular.ucsd.edu/
Intracranial Diseases
Seizure Disorders
Seizure disorders in cats present a significant diagnostic challenge and may result from primary intracranial disease or extracranial disease. Seizures can be classified as focal, partial, or generalized.34 A focal seizure is one in which there is spontaneous discharge of neurons of the prosencephalon in the absence of clinical signs and that may be present in the interictal period but is detectable only with use of an electroencephalogram (EEG). A partial seizure is a focal seizure that may be observed clinically and consists of varying degrees of motor or sensory abnormalities in the absence of loss of consciousness. A simple partial seizure results in abnormal motor activity such as twitching, tremors, limb flexion, ptyalism, facial twitching, and mydriasis with no alteration in sensorium. A complex partial seizure may resemble the simple partial seizure, but changes in the mental status are evident, including maniacal running, staring into space, aggression, and self-inflicted trauma.34 Generalized seizures (grand mal) are more easily recognized by pet owners and result in loss of consciousness, recumbency, tonic–clonic muscle activity in the limbs, chewing movements, ptyalism, mydriasis, and loss of stool and urine.34 The seizure type does not necessarily reflect underlying etiology,114 although seizure pattern in cats is reportedly different from that of dogs, with complex partial seizures being more common than generalized seizures.109 Cluster seizures are defined as more than two seizures in a 24-hour period, whereas status epilepticus (SE) is a seizure lasting more than 5 minutes or multiple seizures between which there is no recovery.34 Structural brain disease has been identified as the most common cause of seizures in cats and includes meningoencephalitis, feline ischemic encephalopathy, neoplasia, trauma, abscess, and vascular disorders.4,109 However, idiopathic epilepsy (defined as recurrent seizures in the absence of an underlying cause) is an important and often overlooked cause of seizures in cats, accounting for 25% of cases in one report of 91 cats.114 Cats with idiopathic epilepsy tended to be younger than those with structural brain disease, with a mean age of 3.5 years in two publications.114,126 Feline hippocampal necrosis should also be considered in the differential diagnosis for a seizuring cat. It is characterized by acute onset seizures and behavioral changes in young to middle-aged cats with poor response to conventional anticonvulsant therapy and progressively worsening signs.40 Histopathologic findings include severe, diffuse, bilateral necrosis of neurons in the hippocampus and piriform lobes.40 Prognosis is guarded.
Diagnosis
Evaluation of affected cats requires a thorough history and neurologic exam, as well as consideration of the breed, age, and vaccine history of the patient. A thorough description of the seizure as well as duration, frequency, and the presence or absence of interictal abnormalities are vital to formulate a diagnostic plan. An initial minimum database should include a complete blood count, chemistry panel, blood pressure measurement, urinalysis, and testing for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). Thoracic radiographs, abdominal ultrasonography, echocardiography, bile acid assessment, and thyroid testing may all be part of the workup, depending on the results of the physical examination, history, and preliminary testing. If a metabolic or systemic cause is ruled out through the preliminary workup, advanced imaging and cerebrospinal fluid (CSF) analysis is indicated. Magnetic resonance imaging (MRI) is the most reliable imaging modality for the diagnosis of structural diseases of the brain on account of its superior anatomic detail.4,34
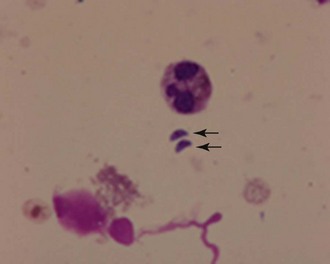
CSF analysis is an invaluable resource in the diagnosis of many primary encephalopathies, but findings are rarely specific and results must be interpreted in light of clinical and MRI findings. CSF analysis infrequently provides a definitive diagnosis, unless infectious agents such as Cryptococcus (Figure 27-1) or neoplastic cells are identified. In one report, despite extensive evaluation, a definitive diagnosis could not be made after CSF analysis in 37% of cats.17
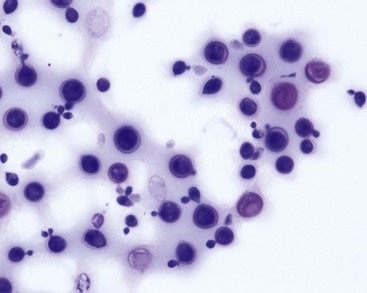
FIGURE 27-1 Cryptococcus organisms found in cerebrospinal fluid. (Wright’s stain.)
(From Cowell RL, Tyler RD, Meinkoth JH et al, editors: Diagnostic cytology and hematology of the dog and cat, ed 3, St Louis, 2008, Mosby Elsevier.)
Collection techniques require a thorough understanding of neuroanatomy as well as application of the proper technique.17 In most cats the cerebellomedullary cistern is the site of choice, although lumbar collection may be preferable in cats with a thoracolumbar myelopathy.
A 22-g, 1.5-inch spinal needle with a stylet is appropriate for most cats. General anesthesia is induced, and the desired site of collection is clipped and aseptically prepped. For collection from the cerebellomedullary cistern, the cat is placed in right lateral recumbency for a right-handed clinician with the neck flexed. The wings of the atlas and occipital protuberance are palpated and an imaginary line drawn between the wings of the atlas and the point where this line transects midline. The spinal needle is inserted slowly, with frequent removal of the stylet to evaluate CSF flow. When the subarachnoid space is entered, the CSF (at least 1 mL) can be allowed to passively drip into a sterile glass tube.17
Treatment
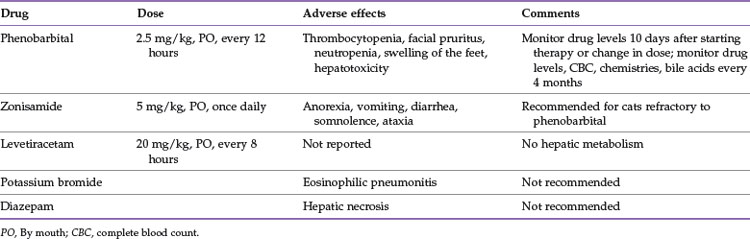
Treatment is directed at the underlying disease and pharmacologic management of the seizures (Table 27-1). Phenobarbital (PB) is the drug of choice for most feline seizure disorders because of its efficacy, relative safety, ease of administration, and bioavailability.34,133 A starting dose of 2.5 mg/kg, administered orally every 12 hours, is recommended,133 but serum PB concentrations vary greatly among individuals on the same dosage, which suggests that there are differences in elimination kinetics among populations of cats.23 These differences emphasize the need for individual monitoring of cats receiving PB,23 and researchers recommend more frequent monitoring of PB levels in cats compared with dogs.108 Elimination of PB may be accelerated in cats receiving steroids and in kittens, which suggests a probable need for higher dosing in these individuals.108 Toxicity is extremely unusual in cats receiving therapeutic doses but may include thrombocytopenia, facial pruritus, neutropenia, or swelling of the feet.108 Hepatotoxicity is equally rare. The author suggests checking PB levels approximately 10 days after initiation of therapy or after a change in dose, as well as every 4 months along with a complete blood count, chemistry panel, and assessment of bile acids. Samples can be drawn at any time during the day.
Potassium bromide (KBr) is commonly used in dogs as an add on to PB or in patients with hepatic disease. Although relatively effective for seizure control (seizures were eradicated in 7 of 15 cats in one report), KBr has fallen out of favor for cats because of the high incidence of drug-induced eosinophilic pneumonitis associated with its use.13 Similarly, diazepam, once considered the second line of therapy for controlling seizures in cats, is not recommended owing to the risk of fatal hepatic necrosis associated with oral administration.18 Zonisamide, a newer anticonvulsant medication, has been used by the author in cats refractory to PB with promising results. Toxicity is reportedly low, but approximately half the cats in one study developed adverse reactions such as anorexia, vomiting, diarrhea, somnolence, and ataxia.52 Further studies are needed regarding efficacy and pharmacokinetics of this drug, but anecdotally, a starting dose of 5 mg/kg, administered orally once daily, has proved effective in a number of cases.
Levetiracetam, another relatively new drug, has been shown to be an effective and safe drug when used as an adjunct to PB therapy in cats with idiopathic epilepsy.3 Adverse effects were not reported, and 7 of 10 cats treated experienced a greater than 50% reduction in seizure frequency and none of the cats experienced SE after beginning the drug.3 It is noteworthy that there appears to be no hepatic metabolism. The suggested starting dose is 20 mg/kg, administered orally every 8 hours.
Prognosis for seizure disorders depends on the underlying cause and is discussed for individual diseases in detail in the following section. It is important to note that severity of seizures does not predict prognosis,108 and cats without other neurologic abnormalities may have excellent outcomes with aggressive management.4,108
Degenerative Disorders
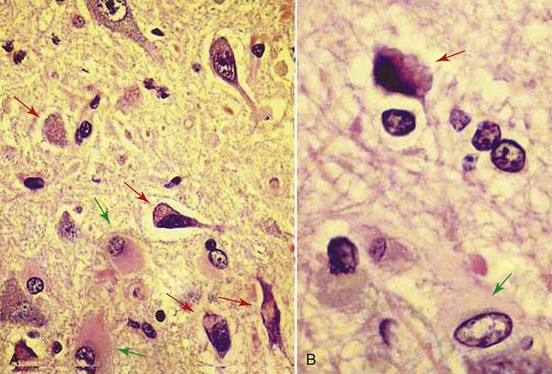
Lysosomal storage diseases are genetic disorders resulting in accumulation of large cytoplasmic inclusions containing undigested products of cellular metabolism (Figure 27-2).30 Most are inherited as an autosomal recessive trait and result in a deficiency or malfunction of key enzymes within the lysosomal catabolic pathway.125 Storage diseases are highly diverse and have been organized into subgroups (on the basis of the deranged metabolic pathway), including the glycoproteinoses, the oligosaccaridoses, the sphingolipidoses, the mucopolysaccharidoses (MPS), and the proteinoses.125 Features common to the storage diseases include equal distribution between males and females, a slowly progressive clinical course (with the cat being normal at birth and in the first few months of life), and in some cases a history of neonatal deaths within the litter.30 The neurologic signs can be highly diverse depending on the specific disease; multiple organ systems and all levels of the nervous system may be affected. However, the predominant clinical signs usually begin with cerebellar or cerebellovestibular impairment.125 In some disorders, such as Niemann–Pick disease type A or globoid cell leukodystrophy (GCL), signs of a peripheral neuropathy may predominate. Similarly, ceroid lipofuscinosis, reported rarely in Siamese cats, may present with primarily prosencephalic signs, including seizures and blindness.125
Diagnosis
Diagnosis of a storage disease can be challenging, and an index of suspicion may be raised if an at-risk breed is involved or if there is a history of prior progeny of the same parents being similarly affected.30 Results of routine hematologic and biochemical testing is usually normal in these patients, although careful examination of blood smears may alert the clinician to the presence of storage vacuoles within leukocytes.125 Biopsy of lymphoid tissue, including the spleen, or liver may reveal evidence of vacuolation.125 In some subgroups, such as MPS, connective tissue abnormalities are common and radiography may reveal skeletal abnormalities, particularly of the spine. Muscle biopsies may reveal pathologic changes, particularly with glycogen storage diseases, and peripheral nerve biopsies are advocated in the diagnosis of GCL.125 Urine metabolic screening is available (Josephine Deubler Genetic Disease Testing Laboratory, University of Pennsylvania) to identify the presence of urinary excretory products, with characteristic excretory profiles being described for specific diseases.125 A definitive means of diagnosing a storage disease is through the use of lysosomal enzyme analysis, in which activity of select lysosomal enzymes in the affected cat are measured against an age-matched control. Affected animals typically have 0% to 5% of the normal activity of the enzyme in question, whereas carrier animals have approximately 50% of normal activity.125 Molecular genetic testing will become increasingly available in the coming years as another means of diagnosing this group of disorders; currently, DNA testing is available only for MPS 6 in cats.125
Anomalous and Congenital Disorders
Malformations of the brain are not uncommon in veterinary patients, with the majority resulting from hereditary causes and in utero exposure to toxins or infectious agents.34 Infectious agents lead to both hypoplasia after destruction of progenitor cells and atrophy secondary to destruction of differentiated actively growing tissue.34
Hydrocephalus
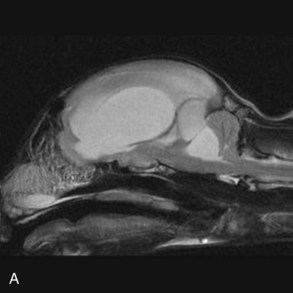
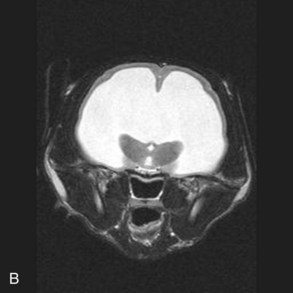
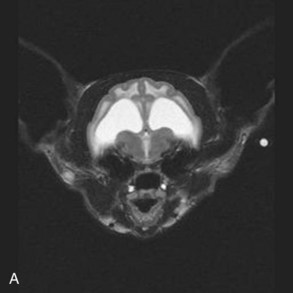
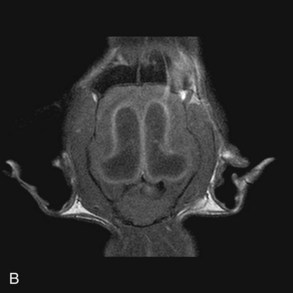
Hydrocephalus is one of the more commonly recognized congenital central nervous system (CNS) disorders in young cats, with clinical signs becoming apparent in kittens as young as 2 to 3 months of age.22 It is characterized by an increase in volume of CSF caused by compensatory or obstructive mechanisms leading to varying degrees of dilation of the ventricular system.34 Enlargement of the cranium, small stature, an open fontanelle, calvarial distortion, divergent strabismus, abnormal behavior, ataxia, seizures, visual deficits, head pressing, and stupor may all be sequelae to hydrocephalus, depending on the severity of the pathologic changes in the brain.22 Advanced imaging is necessary to confirm the diagnosis and rule out other causes of intracranial disease. Typical findings on MRI (Figure 27-3) include varying degrees of ventricular dilation, reduction of periventricular white matter, expansion of the cranial cavity, and loss of cortical bone.
Treatment of congenital hydrocephalus may include medical management with the aim of reducing CSF volume and production through the use of diuretics and corticosteroids.22 Furosemide at a dose of 0.5 to 4.4 mg/kg PO, IV, IM, every 12 to 24 hours, may be used to decrease CSF production through inhibition of the sodium–potassium cotransport system.22 Acetazolamide (Diamox) may be used in a similar fashion at 10 mg/kg PO, every 8 hours; it acts to decrease CSF production through inhibition of carbonic anhydrase.22 Prednisone may also be administered to reduce production of CSF, but medical management usually provides only temporary relief of clinical signs.22 Surgical intervention requires placement of a ventriculoperitoneal or ventriculoatrial shunt, with prognosis dependent on the severity of the underlying disease.22
Cerebellar Hypoplasia
Cerebellar hypoplasia is a well-recognized syndrome in cats resulting from in utero or perinatal exposure to feline panleukopenia virus.34 The virus has a predilection for rapidly dividing cells and targets the external germinal layer of the cerebellum. As such, hypoplasia of the granule layer and disorganization of the Purkinje cells results (Figure 27-4), leading to varying degrees of impairment.34 Clinical signs become evident as soon as the animal is able to walk and include a base-wide stance, coarse whole body tremors, intention tremors, cerebellar quality ataxia, and hypermetria. Neurologic deficits are symmetric and nonprogressive in nature.34 Depending on severity of disease, an affected kitten can have a good quality of life, provided adequate measures are taken to prevent injury from falling and to keep it indoors. This disorder is best prevented by vaccinating queens before pregnancy.34 Although similar clinical signs are seen in cats with cerebellar abiotrophy, the two disorders can be readily distinguished given that signs resulting from abiotrophy usually do not become apparent until several months to years of age and are progressive in nature.6
Miscellaneous Anomalies
Other congenital anomalies are less well recognized and occur sporadically from genetic, toxic, or infectious causes. Meningoencephalocele has been well recognized in Burmese kittens as part of an inherited craniofacial malformation and has also been associated with in utero exposure to griseofulvin.34 Intracranial arachnoid cysts have been reported to arise in the quadrigeminal cistern in Persian cats, with clinical signs including obtundation and collapse.80,110 Lissencephaly with microencephaly in Korat cats has been reported and is associated with signs of abnormal behavior and self-mutilation.
Metabolic and Nutritional Encephalopathies
Numerous metabolic diseases result in neurologic signs in cats through effects on the metabolism of neurons in the CNS.34 Hypoglycemia is a well-recognized cause of seizures in pediatric patients and has also been reported infrequently in older cats with insulinoma or other insulin-secreting tumors.50,67 Additional causes include hepatic disease, sepsis, lysosomal storage diseases, inadvertent overdose of insulin, and hypoadrenocorticism.67 Clinical signs associated with neuroglycopenia in addition to seizures may include lethargy, weakness, disorientation, ataxia, and visual deficits. Chronic hypoglycemia may cause irreversible nerve damage in cats,67 and thus prompt treatment aimed at correcting the underlying problem is essential.
Hepatic encephalopathy is most often seen in young cats with portosystemic shunts (PSSs) and has also been recognized in other hepatic disorders (e.g., lipidosis, damage caused by hepatotoxic drugs). Toxic products that are released from the gut normally are detoxified by the liver, but in affected cats increased levels of ammonia, benzodiazepine-like substances, and other metabolites circulate to the brain.34 Resultant clinical signs include seizures (often postprandial), circling, depression, ptyalism, blindness, head pressing, disorientation, and poor growth.145 Gastrointestinal and urinary signs as well as poor growth often accompany neurologic deficits. A complete description of diagnosis and treatment of PSS and other hepatopathies can be found in Chapter 23.
Various endocrinopathies and electrolyte abnormalities have also been associated with intracranial signs. Diabetic ketoacidosis and diabetic hyperosmolar nonketotic syndrome may produce neurologic dysfunction leading to signs of lethargy, depression, anorexia, and stupor.138 Coma may result from dehydration of brain cells secondary to chronic hypovolemia, osmotic diuresis, and shifts in fluid balance between the intracellular and extracellular compartments.138
Hyperthyroidism has been reported to cause restlessness, hyperexcitability, pacing, circling, anxiety, and mental confusion.61 Seizures may also be seen, either as a direct result of thyroid hormones decreasing the electric threshold of cerebral tissue or related to a vascular accident secondary to hypertension.
Naturally occurring hypoparathyroidism results in severe hypocalcemia and results in focal or generalized muscle tremors, seizures, ataxia, disorientation, stilted gait, lethargy, anorexia, and elevated nictitans. Other causes of hypocalcemia include renal disease, ethylene glycol toxicity, pancreatitis, eclampsia, phosphate-containing enemas, and iatrogenic causes related to thyroidectomy.41
Hypercalcemia can cause disturbances of the CNS such as depression and seizures and is most often associated with hypercalcemia of malignancy or renal failure, although there are rare reports of primary hyperparathyroidism in cats.41
Hypernatremia, defined as a serum sodium concentration above 165 mEq/L in cats, results in clinical signs of weakness, ataxia, seizures, and coma.86 The severity of clinical signs is related directly to the acuteness of onset and degree of hypernatremia86 and is attributed to rapid shifts of water from the intracellular to the extracellular space.48 Rapid correction with inappropriate fluid therapy can lead to severe complications, including cerebral edema and death.48
Thiamine-deficiency encephalopathy is well recognized in cats and is characterized by vestibular-quality ataxia, mydriasis, cervical ventroflexion, and seizures.30 Affected cats often have a history of eating a raw-fish diet, which is rich in thiaminase.30 The author has observed a number of cats with presumed thiamine deficiency in which clinical signs were preceded by prolonged anorexia, such as with hepatic lipidosis. Administration of thiamine (10 to 25 mg administered intramuscularly and followed by oral supplementation) will result in complete resolution of clinical signs. If the condition is left untreated, signs progress to prostration, opisthotonos and spasticity, coma, and death.30 On postmortem examination, petechial hemorrhages are found bilaterally in the brain stem, with degenerative lesions found in the caudal colliculi, lateral geniculate, vestibular, oculomotor, and habenular nuclei.30
Neoplasia
Brain tumors occur in cats with an overall incidence of 3.5 cases per 100,000 cats and account for 2.2% of all tumors.76 Primary brain tumors include meningioma, glioma (astrocytoma, oligodendroglioma), ependymoma, choroid plexus tumor, and rare embryonal tumors (e.g., neuroblastoma, primitive neurectodermal tumors, medulloblastoma).139 Secondary tumors include pituitary tumors, tumors that invade by direct extension into the brain (e.g., nasal tumors, otic tumors, ocular tumors), and metastatic tumors (e.g., mammary adenocarcinoma).76 In a retrospective study of 160 cats with intracranial neoplasia,141 tumor prevalence was determined to be as follows: 58.1% meningioma, 14.4% lymphoma, 8.8% pituitary, 7.5% glioma, 5% neuroepithelial, 5.6% metastatic, and 3.8% direct extension. There is no reported breed predisposition in cats, and the median age for developing a brain tumor is 11 years.139
Intracranial tumors infiltrate the parenchyma of the brain, leading to disruption of blood flow, cerebral edema, local necrosis, disruption of CSF flow leading to obstructive hydrocephalus, and increased intracranial pressure (which can result in herniation).99 Primary intracranial tumors rarely metastasize, but in some cases they may spread to the lungs by drainage through the venous sinus plexuses in the cranial vault.99 Clinical signs can include behavioral changes, circling, seizures, visual deficits, ataxia, and paresis115; however, signs can be nonspecific, and in one report 21% of cats presented only for anorexia and lethargy.136 Signs are often slowly progressive and asymmetric in nature; however, in some asymptomatic patients tumors may be found as incidental findings at necropsy.141
Meningiomas
Meningiomas are the most common primary brain tumor in cats and are mesenchymal in origin, arising from the arachnoid layer of the meninges. The majority of cases involve older patients34,93 with males slightly overrepresented at a ratio of 3:2.33 Their topography is similar to that of dogs, with most being supratentorial and frequently involving the third ventricle.33,140 There is a tendency in cats for these tumors to be multiple, and in one report 19% of cats had more than one meningioma.148 In another report three cats had two meningiomas, and another cat had four meningiomas.43 The presence of multiple lesions may result in multifocal signs, confounding the clinical picture and differential diagnosis. However, despite multiple lesions, 75% of cats in one study presented with signs suggestive of a focal lesion.43
Microscopically, most feline meningiomas are meningotheliomatous or psammomatous, and many have cholesterol deposits.33 Clinical signs depend on the location and rate of growth of the tumor but are usually insidious in onset because of their slow growth rate.115 The median duration of clinical signs before presentation was 1.25 months in a retrospective of 42 cats and included mentation changes (100%), visual deficits (93%), paresis (83%), and seizures (19%).49
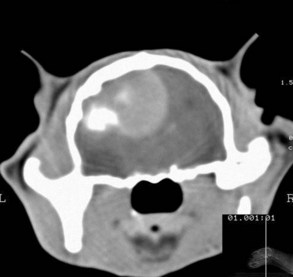
Definitive diagnosis ultimately requires histopathologic analysis, but advanced imaging (computed tomography [CT], MRI) can be highly suggestive of meningioma on account of the characteristic imaging features and anatomic location. CT is not as useful for detection of intracranial masses as MRI because of its poor soft tissue detail and lack of ability to identify lesions in the caudal fossa. However, in a study of canine brain tumors, CT correctly predicted histologic type in 86% of cases.107 Meningiomas appear isodense to hyperdense, homogenous, and brightly enhanced on CT, and calcification is easily recognized (Figure 27-5).115 Hyperostosis, frequently seen in the calvarium overlying feline meningiomas, is readily detected by CT scan and is reported to occur in approximately 73% of cases.
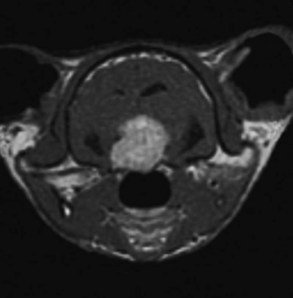
MRI is considered the superior modality for detecting these tumors and correctly identified meningioma on the basis of MRI features alone in 96% of cases in 33 cats.140 Imaging features are variable but include an extraaxial location, distinct margins, mild peritumoral edema, mass effect, dural tail, and broad base (Figure 27-6).140 In one report enlargement of the lateral ventricle was present in 64% of cases, and herniation under the tentorium or cerebellum was evident in 63% of cases.140 Results of CSF analysis in cats with meningioma have not been reported, but in dogs they are nonspecific and unlikely to be of diagnostic benefit.
The mainstay of treatment for feline meningioma is surgical removal. Surgery is often successful owing to the superficial location over the cerebral convexities and frontal lobes, the lack of invasion of underlying parenchyma (unlike dogs), and the well-circumscribed nature of these generally benign tumors. Postoperative mortality rates have been reported to be as high as 19%,49 but in the author’s experience and that of other experienced clinicians,115 the mortality rate is typically lower. The most common postoperative complication after meningioma excision is anemia, which occurs in as many as one third of cats.49 Overall, median survival time (MST) in a study of 42 cats was 26 months, with a 1-year survival rate of 66% and a 2-year survival rate of 50%.49 In the same report 30% of cats developed a recurrence of neurologic signs, with a median time of 30.75 months. In another report of 34 cats with surgically treated meningioma, MST was 685 days, with 20% of cats experiencing a recurrence of clinical signs (285 days). Data regarding treatment of feline meningioma with radiation or chemotherapy are lacking, probably because of the high success and long survival times after surgery.
Pituitary Tumors
Pituitary tumors are rarely diagnosed in cats and often exert their clinical effects through excessive secretion of growth hormone. Clinical signs include acromegaly, characterized by enlargement of the head, lameness, dyspnea resulting from cardiomegaly, protrusion of the mandible, respiratory stridor caused by hypertrophy of oropharyngeal soft tissues, and widening of the interdental space.128 Severe insulin-resistant diabetes is often evident. However, neurologic signs (e.g., seizures, behavioral changes, blindness) may occur in the absence of an endocrinopathy.62,88 Middle-aged to older male cats are overrepresented.88,128
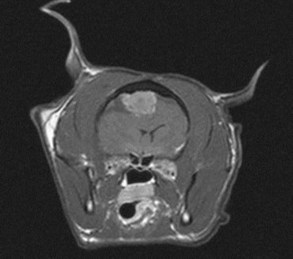
Definitive diagnosis requires advanced imaging of the brain (CT or MRI) and usually reveals a mass lesion in the sella with dorsal expansion into the overlying diencephalon (Figure 27-7). The majority of macroadenomas are contrast enhancing and may have areas of necrosis, hemorrhage, or calcification.
Medical management of these tumors is largely unrewarding, and more definitive treatment requires surgical intervention or radiation. In seven cats treated with transphenoidal hypophysectomy, surgery was well tolerated in most cases, with clinical signs resolving in five cats and two cats surviving 28 and 46 months after surgery.89 However, surgeons competent in this procedure are lacking, and complications, including chronic oronasal fistula, can have a significant impact on quality of life.89 Treatment with radiation therapy has been shown to ameliorate or resolve clinical signs and has been associated with prolonged survival times in numerous studies.62,88 Median survival time in a report of eight cats treated with radiation was 17.4 months (range, 8.4 to 63.1 months).62
Lymphoma
Lymphoma of the brain can be primary or secondary or may be an aspect of multicentric disease.76 It is seen uncommonly, accounting for only 14.4% of cases in a series of 160 cats with intracranial tumors.141 In a retrospective of 18 cats with CNS lymphoma, 14 had intracranial involvement and 10 presented with a chief complaint of seizures.98 Most important, the prevalence of involvement of bone marrow and other organs was extremely high, suggesting that perhaps the most reliable means of diagnosing lymphoma in the CNS is through confirming its presence in other body systems.98
There are no pathognomonic findings on MRI of cats with intracranial lymphoma, and in some cases imaging features are similar to those of meningioma.140 CSF analysis may be highly useful, and malignant cells were seen in the CSF of 5 of 11 cats in one report98 and 6 of 17 in another.72
Although lymphoma is considered chemotherapy sensitive, prognosis in affected cats is guarded, with a MST of approximately 21 days in patients treated with prednisone alone.141 Chemotherapy alone has not proved to substantially prolong survival times, but when combined with radiation, the MST was found to be 125 days (range 40 to 210 days).98
Other Tumors
The incidence of other tumors of the brain in cats is not known, but they are seen only rarely in clinical practice. Of 160 cats with intracranial tumors, astrocytoma, oligodendroglioma, olfactory neuroblastoma, and ependymoma accounted for only 7.6% of cases. Because of the rarity of these tumors, there is a paucity of information regarding treatment and prognosis. However, gliomas typically appear to carry a grave prognosis, and in a cat with an astrocytoma treated with surgery and radiation, the survival time was only 179 days.141 Ependymomas carry a more favorable prognosis and seem to respond well to surgical intervention, with survival times as long as 2 years reported.124,141
Inflammatory Disorders
Feline Infectious Peritonitis
Feline infectious peritonitis (FIP) is the most common and clinically significant inflammatory disorder in the CNS, accounting for 48% of cases of infectious neurologic diseases reported in cats.14 The causative agent, a highly pathogenic variant of feline enteric corona virus (FIPV), produces immune-mediated disease through infection of macrophages, with severity of signs determined by host susceptibility, host-specific immune response, and virus strain.42 The majority of cases are younger than 2 years of age and come from multicat households, with males and purebreds being overrepresented.35,42
Neurologic signs can be seen with both the effusive (“wet”) and the noneffusive (“dry”) form of this disease, but the dry form appears more commonly to involve the CNS.35 Signs referable to cerebellomedullary involvement are most common,32 but seizures may also be evident and have been reported in up to 25% of cats with histopathologically confirmed FIP.135 Ataxia, spastic paresis, head tilt, nystagmus, hyperesthesia, proprioceptive deficits, blindness, and behavioral changes have all been reported.35,42 Non-neurologic signs frequently accompany the CNS signs and include uveitis, chorioretinitis, respiratory infections, mesenteric lymphadenopathy, dehydration, weight loss, lethargy, fever, and pica.42
Antemortem diagnosis of FIP can be extremely challenging and requires a high index of suspicion, especially in those patients with no obvious systemic involvement (see Chapter 33). Complete blood count findings are nonspecific, but serum protein concentration is often elevated, specifically the alpha-2, beta, and gamma-globulins.35 CSF analysis in cats with FIP is characterized by increased cellularity (which may be predominantly neutrophilic or mononuclear), as well as increased protein levels as high as 2 g/L. The presence of anti-coronavirus antibodies in either serum or CSF proves only that the cat has been exposed to a coronavirus and is not a means of definitively diagnosing FIP. In a prospective study of 67 cats, detection of anti-coronavirus antibodies in CSF had a sensitivity of 60% and a specificity of 90%.11
MRI is a useful adjunctive tool in cases of suspected intracranial FIP, both to help confirm the diagnosis and rule out other causes of neurologic signs (Figure 27-8). Typical findings include ventricular dilation, ependymitis, choroid plexitis, meningitis (most evident on the ventrocaudal surfaces of the brain), cervical syringomyelia, and periventricular inflammation.42,100 However, there are no pathognomonic imaging findings, and results must be considered in light of clinical and clinicopathologic findings. PCR can be performed on CSF and other fluids (e.g., abdominal effusion) or tissues, but its sensitivity is relatively low, and a negative test does not necessarily rule out FIP.42
Postmortem examination of the brain often reveals gross lesions, including meningeal opacity around the medulla and choroid plexus of the fourth ventricle and coating of the choroid plexuses with a white tenacious exudate.32 Ependymal cells may be coated by fibrin and may lead to hydrocephalus rostral to the obstruction. Histologically, there is a severe pyogranulomatous leptomeningitis, choroiditis, ependymitis, and encephalomyelitis, with lesions predominantly surface oriented.32 Prognosis is extremely grim, and despite claims of effective treatments for FIP, it appears that the disease is uniformly fatal, even with supportive care and immunomodulatory therapies.
Toxoplasmosis
Toxoplasma gondii is a protozoal parasite for which the cat can serve as both the intermediate and definitive host.32,79 Infection occurs by direct ingestion of tissue cysts in meat or sporulated oocysts from cats feces, as well as transplacentally.32 After infection bradyzoite organisms will become encysted in various tissues, including muscle and CNS, but often the infection remains latent and the patient will be asymptomatic.32 Clinical disease occurs with a variety of immunocompromising factors, including steroid administration, concurrent infection with FIV or FeLV, stress, a large infective dose in very young animals, and neoplasia.32,79
Clinical findings include fever, pneumonia, icterus, abdominal discomfort, dyspnea, pericardial effusion, ascites, pancreatitis, and mesenteric lymphadenopathy.79,134 Lesions in the CNS are uncommon, accounting for only 7 of 100 cats with histologically confirmed toxoplasmosis.36a Clinical signs of CNS involvement are often multifocal and include seizures, blindness, ataxia, abnormal behavior, depression, anisocoria, head tilt, and nystagmus.36a,79
Antemortem diagnosis can be challenging and use of an IgG-based test (e.g., enzyme-linked immunosorbent assay [ELISA], latex agglutination test) requires demonstration of a fourfold increase in IgG over 2 to 3 weeks.79 CSF can be used to determine intrathecal antibody production, but results must be interpreted cautiously because T. gondii–specific IgG has been found in the CSF of normal cats.79 PCR has also been used to detect T. gondii in CSF, and results were found to be positive in seven of seven cats with immunohistochemical or serologic evidence of toxoplasmosis.113 In rare cases organisms may be seen directly in CSF (Figure 27-9) or other biologic material, such as fluid obtained through bronchial lavage.
Histologically, nonsuppurative meningoencephalitis affecting gray and white matter is seen with occasional periventricular involvement.32 Necrosis may be severe, especially in congenital infections, and organisms may be visualized at the margins of lesions, within macrophages, and in tissue cysts.32 Treatment may yield a favorable response, and clindamycin (10 to 12 mg/kg, administered orally every 12 hours for 4 weeks) is considered the drug of choice.79 Alternatively, trimethoprim sulfa (15 mg/kg, administered orally every 12 hours for 4 weeks) can be used.
Fungal Infections
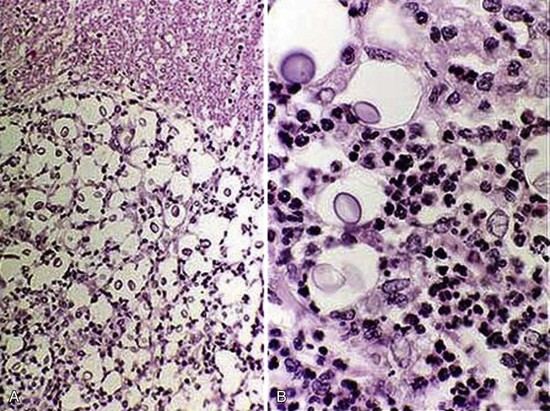
Fungal infections are occasionally identified in the CNS in cats, with Cryptococcus neoformans being the most commonly reported. Because cats with cryptococcosis frequently are infected through inhalation of unencapsulated organisms, it is not unusual for the cat to have concurrent upper respiratory signs as well as swelling of the nose.74 Clinical signs usually reflect a multifocal process, but forebrain signs may predominate because of the proposed route of entry. Ocular involvement often accompanies lesions in the CNS, with organisms being found between the choroid and the retina.32 There is no significant age or sex predilection, and both indoor and outdoor cats can become infected.
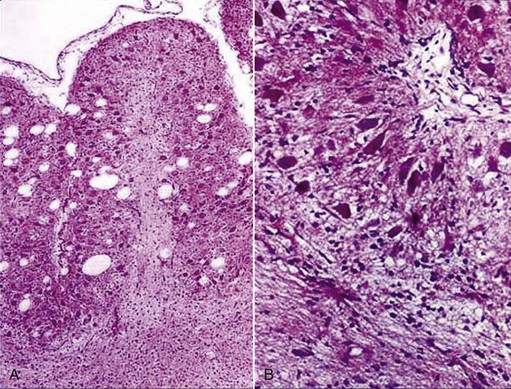
Definitive diagnosis can be obtained with the latex agglutination (LA) test for capsular antigen, a test that is both highly sensitive and specific. LA may also be performed on CSF and may be preferable in cats without obvious systemic involvement. The organism may also be directly visualized in CSF, nasal exudates, skin lesions, urine, and lymph node aspirates.74 In cases in which the organism is not seen in CSF, it is generally still abnormal and may yield neutrophilic, eosinophilic, mononuclear, or mixed pleocytosis with elevated protein.74 MRI of affected cats is variable and may include a solitary granuloma, multifocal masses, meningeal inflammation, and enhancement of the ependyma and choroid plexuses.130 Microscopic findings include the presence of numerous, tightly packed organisms filling the subarachnoid space and expanding the sulci with a mild nonsuppurative inflammatory response in the meninges and parenchyma (Figure 27-10).32
The treatment of choice is currently fluconazole (25 to 50 mg orally every 12 hours) because of its ability to cross the blood–brain barrier, its relative safety margin, and its reported efficacy.74 However, prognosis is considered extremely guarded in cats with CNS involvement and relapses are common. Other fungal infections, such as Blastomyces dermatitidis, Histoplasma capsulatum, and Cladophialophora spp., are sporadically reported in endemic areas and are typically associated with a grave prognosis.74,127
Borna Disease
Borna disease virus (BDV) is the cause of a severe nonsuppurative encephalomyelitis reported in many parts of the world, particularly Europe and Australia. It is most often seen in rural cats prone to hunting birds and rodents and is characterized by pelvic limb ataxia followed by mentation changes, visual deficits, photophobia, circling, and seizures.9 Clinical signs last from 1 to 4 weeks and usually result in progressive impairment and death, although in some cases recovery is possible. Cats that have recovered have permanent ataxia, behavioral changes, and polyphagia.9
Definitive diagnosis can be difficult, and other causes of multifocal CNS disturbances, such as FIP, must be ruled out. Fewer than half of affected cats test positive for BDV-specific antibodies, and PCR has not proved to be a reliable test in this species.9 The disease can be definitively diagnosed only through postmortem examination. Findings include inflammatory changes, mostly in gray matter, perivascular cuffing, neuronophagia, and detection of BDV antigen in the CNS parenchyma.9 Prognosis is grave, and there is no known treatment. Many other infectious encephalitides in cats have been reported uncommonly, including rabies, FIV, rickettsial disease, pseudorabies, and feline spongiform encephalopathy.
Toxic Disorders
Toxin ingestion should be considered in any cat presenting with acute neurologic signs, particularly in those cats with a history of indiscriminate ingestion of foreign materials or those that have had access to the outdoors with no supervision. Because many toxins can produce effects similar to naturally occurring diseases on the CNS, a thorough history, as well as a complete neurologic examination, is essential. Although the list of potential neurotoxins is endless, this section addresses only the most common and clinically relevant ones seen by feline practitioners (see also Chapter 31).25
Topical pesticides represent a significant source of toxicity in cats and usually result from inappropriate administration of flea and tick products. Clinical signs of permethrin toxicity include tremors and muscle fasciculations, twitching, hyperesthesia, seizures, ataxia, mydriasis, and central blindness. Severe clinical signs require intensive treatment, and left unchecked, death from aspiration pneumonia, respiratory arrest, or electrolyte abnormalities may result. However, the majority of cats will have a good outcome with no long-term complications.12,37 Organophosphate and carbamate insecticides act by inhibiting acetylcholinesterase, resulting in signs consistent with a “cholinergic crisis” marked by muscarinic, nicotinic, and mixed signs.36 Tremors, depression, seizures, miosis, abnormal behavior, and cervical ventroflexion all have been reported in affected cats within minutes to hours after exposure.36
A number of drugs have been reported to cause neurologic signs in cats, the most well described being metronidazole.16,112 Neurologic signs include disorientation, ataxia, central blindness, and seizures. All reported cases have been on doses greater than 30 mg/kg per day. Withdrawal of the medication and institution of supportive care result in rapid resolution of clinical signs within several days.16,112 Ivermectin is also reported to cause seizures, as well as ataxia, blindness, mydriasis, coma, disorientation, and death.
Plant toxicities account for approximately 10% of reported exposures to poison control centers, with more than 50% of cases being less than 1 year of age.58 These are often particularly challenging to the clinician because many pet owners do not know the names of the plants the cat may have ingested, and in some cases it is unclear whether the animal did in fact ingest the plant material. Tobacco, marijuana, and other hallucinogenic plants have all been reported to cause a multitude of signs, from depression to ataxia, seizures, and death.58
Ingestion of lead continues to be an important toxicologic problem in cats, particularly in those living in homes constructed before 1977. Owners of cats with suspected lead poisoning should be questioned carefully to determine whether remodeling is taking place because the grooming habits of cats put them at risk for ingesting lead-containing particles.36 Neurologic signs, including behavioral changes, seizures, blindness, and ataxia usually develop after acute, high-level lead exposure.36 In cats gastrointestinal signs (e.g., vomiting, anorexia, abdominal pain, constipation, and megaesophagus) are more common than neurologic signs. The diagnosis of lead poisoning can be confirmed by measuring a blood lead level greater than 0.22 ppm.36
Although numerous other toxins have the potential to affect the nervous system in cats, a complete discussion is beyond the scope of this chapter; the reader is referred elsewhere for an excellent review of veterinary toxicology.103
Vascular Encephalopathies
Cerebrovascular Accidents
Cerebrovascular accidents (CVAs) are becoming recognized in veterinary medicine with increasing frequency because of the greater availability of MRI. CVAs can be divided into two broad categories: ischemic stroke, in which a vessel is occluded by thrombus or vasospasm, and hemorrhagic stroke, which arises from rupture of blood vessels into CNS parenchyma or subarachnoid space.45 The incidence of CVA in cats is unknown, insofar as the majority of reports in the veterinary literature are based on canine studies. Risk factors include hyperfibrinogenemia, polycythemia, coagulopathies, neoplasia (e.g., intravascular lymphoma), hypertension, multiple myeloma, cardiac disease, infectious diseases (e.g., FIP), renal disease, vasculitis, and others.45,53,55 Postanesthetic cerebellar ischemia has been reported in Persian cats after anesthesia with ketamine.116
Clinical signs reflect the location and extent of the affected area and are usually acute in onset and asymmetric, with minimal progression after the first 24 hours. The cerebellum is the most common site for vascular accidents to occur,19,34 but the cerebral hemispheres and thalamus are also frequently affected. A minimum database in any cat with suspected brain infarction should include a complete blood count, chemistry panel, FeLV and FIV testing, urinalysis, thyroid panel if applicable, coagulation profile if applicable, multiple blood pressure measurements, electrocardiogram, and possible thoracic radiographs and abdominal ultrasound. If cardiomyopathy is suspected, echocardiography should also be performed. Definitive diagnosis requires advanced imaging of the brain, with MRI considered the superior modality for detecting intracranial infarction (Figure 27-11). Findings may vary depending on the amount of time that has elapsed between the onset of the stroke and performance of imaging. Typical abnormalities noted on MRI include a focal, sharply demarcated lesion that is hyperintense on T2 and FLAIR images, hypointense on T1, with a discrete cutoff between normal and abnormal tissue. Mass effect or midline shifting is usually not seen, and there is minimal, if any, contrast enhancement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree