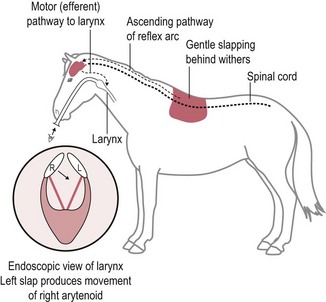
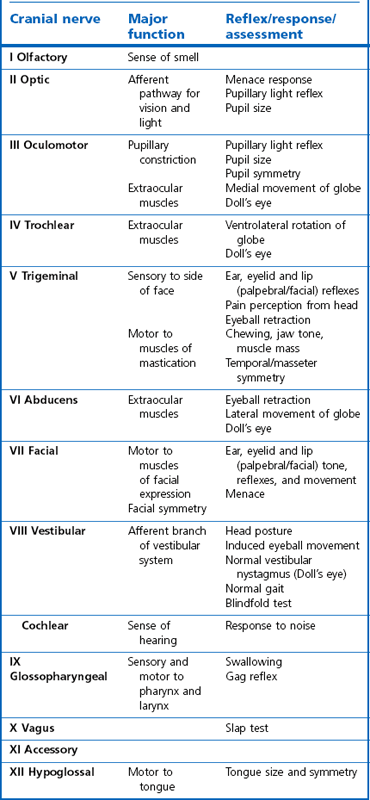
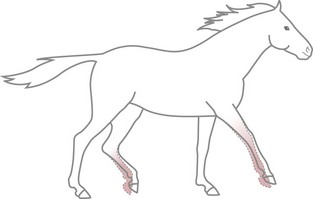
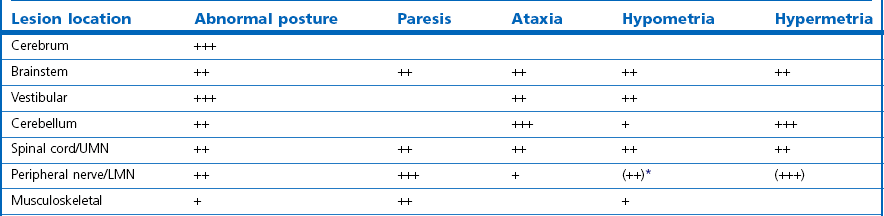
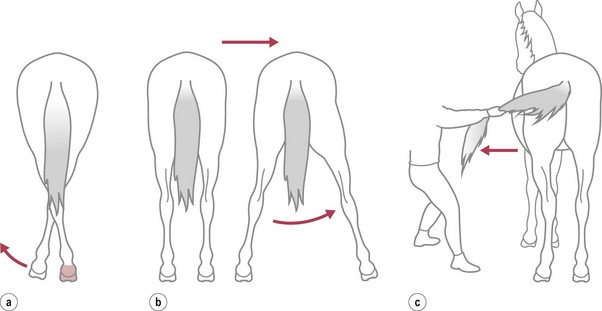
Chapter 11 Perinatal asphyxia syndrome/hypoxic ischaemic encephalopathy/neonatal encephalopathy/neonatal maladjustment syndrome (dummies, barkers, wanderers) 11.13 Equine degenerative myeloencephalopathy (EDM) Peripheral and autonomic nervous systems Important points that should be recorded include: • Age, breed, sex, use; in Quarter Horses: HYPP status. • Duration and progression of signs. • History of trauma or other disease. • Health of in-contact horses. • Diet and recent changes in diet. • Sudden or insidious onset of signs. • General management (vaccination status, travel) and environment. 1. Behaviour, e.g. seizures, head pressing, circling, aggressiveness, etc. 2. Mental status, i.e. level of consciousness and awareness, e.g. coma (complete unresponsiveness to normal stimuli), semicoma (partial responsiveness to stimuli), somnolence, depression, etc. 3. Head posture and coordination, e.g. head tilt, head swaying, jerking. 4. Cranial nerves. The nuclei of the cranial nerves are located along the brainstem, and lesions to the brainstem frequently also result in abnormalities of cranial nerve function. The nerves are examined by starting with the most rostral ones and proceeding caudally. • Olfactory nerve (I) is responsible for the sense of smell. Function of this nerve is difficult to assess, but may be evaluated by testing the horse’s ability to smell the hand or food. • Optic nerve (II) is responsible for vision. The menace response is assessed by making a threatening gesture with the hand to each eye (avoiding air currents or touch) and observing eyelid blinking or movement of the head away. This reflex also relies on cranial nerve VII. Depressed or excited horses, and neonatal foals, may have deficient menace responses. Further assessment of vision may be made by creating an obstacle course. Unilateral vision deficits can be assessed by blindfolding each eye prior to walking through the obstacle course. An ophthalmoscopic examination should be performed in addition to assessment of vision. • Oculomotor nerve (III) is responsible for the control of the pupil (constriction) via parasympathetic fibres (this is opposed by dilator tone controlled by sympathetic pathways). The pupils are assessed for size and symmetry. The pupillary light reflex is performed by shining a bright light into each eye and observing immediate constriction of the pupil in the same eye (direct reflex) and in the opposite eye (consensual reflex). Strabismus (abnormal eye position) may be caused by disease of cranial nerves III, IV or VI, or damage to the extraocular muscles. • Trochlear nerve (IV) is responsible for normal eye position (along with cranial nerves III and VI). Moving the head slowly from side to side causes a rhythmic horizontal movement of each eyeball known as the oculocephalic reflex, doll’s eye, or normal vestibular nystagmus. Extending the head on the neck causes the eyeballs to rotate ventrally within the orbit. The eyeballs return to a central position as the head is lowered. These normal eye movements require an intact vestibular system, intact cranial nerves III, IV and VI, and the connections between these structures. • Trigeminal nerve (V) contains motor fibres to the muscles of mastication, and sensory fibres from much of the head. There are three branches – mandibular, maxillary and ophthalmic. Sensory function is tested by assessing facial reflexes (observed flicking of ear, closure of eyelid, flaring of nostril and withdrawal of the labial commissure in response to touch of these areas); these reflexes also require an intact facial nerve. Facial sensation is tested by observing a cerebral response (e.g. head jerk) in response to pricking areas on the face. Loss of motor function of the trigeminal nerve results in a dropped jaw, and inability or difficulty in chewing, and drooling of saliva. • Abducens nerve (VI) is responsible for normal eye position (with cranial nerves III and IV). • Facial nerve (VII) supplies motor innervation to the muscles of facial expression via the auricular, palpebral and buccal branches. The function of the facial nerve is tested by facial reflexes (which also test the function of sensory fibres of the trigeminal nerve as described above) and observation of the ability to move the ears, blink the eyelids and move the lips during feeding. The facial nerve also innervates the lacrimal and salivary glands. Facial paralysis results in drooping of the ear, ptosis, decreased tear production, and paralysis of the upper lip causing it to be pulled towards the unaffected side. • Vestibulocochlear nerve (VIII) is responsible for hearing (cochlear division) and balance (vestibular branch). Input to the vestibular system also comes from the cerebellum and other brainstem centres. Signs of vestibular disease include a rhythmic nystagmus (fast phase usually directed away from the side of the lesion), strabismus and head tilt (towards the side of the lesion). The nystagmus may occur when the head is in a normal position (resting nystagmus) or when the head is placed in an unusual position (positional nystagmus). Central vestibular disease results in additional signs including proprioceptive deficits, ataxia, weakness and depression. • Glossopharyngeal nerve (X), vagus nerve (XI) and accessory nerve (XI) contain sensory and motor fibres that innervate the pharynx, larynx, oesophagus and many other viscera. The normal swallowing reflex can be assessed by observing the horse eating or drinking, or by passing a nasogastric tube. The pharynx and larynx can be examined by endoscopy. The laryngeal adductor response (slap test) is tested by gently slapping one side of the chest just caudal to the dorsal scapula, while observing the larynx endoscopically. In normal horses the contralateral arytenoid adducts briefly. This reflex involves afferent pathways in the thoracic nerves and cervical spinal cord, and an efferent pathway via the recurrent laryngeal branch of the vagus (Figure 11.1). Pharyngeal paralysis results in dysphagia and nasal return of food. • Hypoglossal nerve (XII) supplies motor innervation to the tongue. Weakness of the tongue is assessed by observing the resistance to pulling the tongue out of the mouth. A summary of cranial nerve function tests is given in Table 11.1. Examination of gait and posture: Abnormalities of gait may arise with either musculoskeletal or neurological problems, and the former must be ruled out before proceeding with the neurological examination. The degree of gait abnormality can be graded on a scale from 0 (normal) to 5 (recumbency). • Ataxia causes an unstable, swaying gait with abnormal foot placement, which becomes worse when the horse is walked on a slope. The limbs may be circumducted, especially on turning. The horse may pivot on the affected limbs when turning or backing. • Paresis causes dragging the feet and stumbling. These signs may be exacerbated by walking the horse in a tight circle or up a slope. • Spasticity causes a stiff movement of the limbs with reduced flexion of the joints. Spasticity can be exaggerated by walking up and down a slope with the head elevated. • Hypermetria results in overstepping with excessive joint movement (Figure 11.2). A summary of the neuroanatomical localization of lesions that result in gait abnormalities is given in Table 11.2. Examination of the neck and forelimbs: • The neck and forelimbs are inspected for symmetry, malformations, muscle atrophy, patchy sweating and the degree and strength of voluntary effort. • Skin sensation and spinal reflexes over the neck and forelimbs are assessed using a pen or probe to prod the skin. This results in flinching of the cervical musculature as well as a behaviour/cerebral response such as head or body movement away from the stimulus. The cervicofacial reflex is tested by prodding caudal to the ear, which results in twitching of the ear, blinking and movement of the labial commissure on the side being tested. • The neck should be manipulated dorsoventrally and laterally to assess the range of movement and presence of pain. • Pushing against the shoulders (sway test) assesses the capacity of the horse to resist lateral force, and is helpful in defining paresis and/or ataxia. Examination of the trunk and hind limbs: • The trunk and hind limbs are observed for musculoskeletal malformations, vertebral column deviations and muscle atrophy. • Skin sensation is assessed as for the neck and forelimbs. Prominent skin flicking over the thorax and flank in response to prodding is known as the panniculus response. • A sway test is performed by providing lateral force to the pelvis (Figure 11.3a,b). A tail pull test is performed by pulling laterally on the tail when the horse is standing still and walking (Figure 11.3c). • A loin pressure test is performed by pressing down firmly with the fingers on the loin and dorsal hip region. Examination of the tail and anus: • The caudal body region is assessed for asymmetry of bone or muscle. Holding the tail elevated or to one side might be normal or abnormal for an individual horse. • Tail tone is assessed by manoeuvring the tail. • The perineal reflex is tested by gently prodding the perineum and observing a reflex contraction of the anal sphincter and clamping down of the tail. • Rectal palpation is performed to evaluate rectal and urinary bladder content and tone. Cerebrospinal fluid (CSF) collection: Atlanta-occipital (AO) space.: With the horse under general anaesthesia, the head is flexed at right angles to the top line of the neck. Using aseptic technique, a needle is inserted at the middle of a line drawn between the cranial borders of the wings of the atlas. The subarachnoid space is entered at a depth of 2.5 to 7 cm with an 18–20 G, 9 cm spinal needle. A palpable ‘give’ in resistance is felt as the subarachnoid space is penetrated. Ten millilitres of CSF can be safely withdrawn. Lumbosacral (LS) space.: Collection from the LS space can be performed in the standing horse. The site of needle insertion is the palpable depression on the dorsal midline just caudal to the sixth lumbar spinous process (between the paired tuber sacrale). This usually coincides with the highest point of the hind quarters. Using aseptic technique, a 15–20 cm, 18 G spinal needle is inserted to a depth of 11–13 cm until a change in resistance is appreciated; the horse often flinches as the subarachnoid space is entered. CSF analysis: Bacteriological and cytological examinations can be performed. Normal CSF is clear and colourless, and has a nucleated cell count less than 6/µL, of which all are mononuclear cells. There are normally no red blood cells, and the total protein is 0.5–1.0 g/L. Radiology: See Chapters 18 and 25. Diagnostic imaging techniques are increasing and improving; currently, in addition to radiography, computed tomography (CT) and magnetic resonance imaging (MRI) modalities may be useful particularly for examination of intracranial disease. In addition, the availability of these techniques is increasing. Electrodiagnostics: Needle electromyography (EMG) can be used to detect disruption of the nerve supply to selected muscles. Damage to motor neurons in the ventral grey column or in peripheral nerves causes abnormalities of the electrical characteristics of the affected muscles. Necropsy: A final diagnosis may be obtained through necropsy; however, this is not always the case. Precautions should be taken to protect people during procedures involving horses with neurological disease lacking antemortem diagnoses, in particular rabies and Borna virus suspects, both zoonotic diseases. Furthermore, the CNS is heavily affected by post mortem autolysis, which can preclude definitive diagnosis of disease. Hydrocephalus is uncommon (Figure 11.4). It may cause dystocia due to gross enlargement of the cranium. Foals are typically non viable, but mildly affected foals may survive and may be affected with a congenital dummy syndrome with slow learning ability. Cerebellar abiotrophy of Arabs: This condition affects purebred or partbred Arabian foals of either sex. The onset of clinical signs is usually between 1 and 6 months of age, but rarely at birth. It is inherited by a recessive gene. There is a presumed deficiency of a vital trophic substance in cerebellar neurons that results in degeneration of neurons with depletion of Purkinje and granular cells. The cerebellum is usually small (less than 8% of total brain weight). • Intention tremors (nodding movements of the head when trying to carry out a specific manoeuvre e.g. moving towards the mare’s udder to suckle). • Deficit of menace response but normal vision. • Hypermetric or hypometric ataxia; some foals show a hypometric gait at a walk that becomes hypermetric at faster gaits; there is no weakness. • Foal may rear and fall over backwards when startled. • Neurological deficits are exaggerated by turning sharply or blindfolding. Sometimes, the clinical signs stabilize, but they usually progress, and euthanasia is required. Other cerebellar conditions: A disorder very similar to the Arabian diesase is seen in Gotland ponies. Also, 1–2 month old Oldenberg foals may show signs referable to a rapidly progressive cerebellar disorder. Occasionally, degenerative cerebellar lesions are seen in newly born Thoroughbred foals when the foal begins to walk or at 2–3 days of age. Seen after poll impact or temporohyoid osteoarthropathy. • Peripheral disease (otitis media or temporohyoid osteoarthropathy): head tilt, circling towards the side of the lesion, recumbency, horizontal or rotatory nystagmus with the fast phase away from the side of the lesion, facial nerve (VII) paralysis. • Central disease: other signs of brainstem involvement, cranial nerve involvement, vertical nystagmus, depression, ataxia and weakness. • Central lesions may result in paradoxical vestibular syndrome: lesion opposite side than expected; other cranial nerve deficits on the side opposite to the direction of circling. • Seizure control: diazepam at 5 mg for a foal and up to 25 mg for an adult should be repeated as necessary. Alternatives: midazolam, phenobarbital, pentobarbital. In some cases, general anaesthesia is necessary (guaifenesin or barbiturates). • Anti-inflammatories: NSAIDs and/or corticosteroids. • Decreasing intracranial pressure: hyperosmolar fluids IV (e.g. 20% mannitol, hypertonic saline (3–7%), perhaps furosemide). • Anti-oxidative stress: dimethyl sulphoxide (DMSO), vitamin E. • Antimicrobials may be indicated. • Surgical decompressive craniotomy is indicated when there are bone fragments penetrating the cerebrum from an open skull fracture. Locoweed and Darling pea toxicity: Locoweed (Astragulus and Oxytropus spp.) in the USA and Darling pea (Swainsona spp.) in Australia can produce a syndrome of dementia and periods of aggression and hyperaesthesia, along with cerebellar ataxia. The alkaloid toxins in these plants induce a lysosomal storage disease (alphamannosidosis) in affected horses. Mouldy corn poisoning – leucoencephalomalacia: Associated with the ingestion of mouldy corn over a period of about 1 month, leucoencephalomalacia (LEM) develops from the mycotoxin fumonisin B1 produced by Fusarium spp., which alters sphingolipid biosynthesis, thus affecting the subcortical white matter. Yellow star thistle poisoning: Nigropallidal encephalomalacia occurs in USA, South America and Australia in horses eating yellow star thistle (Centaurea solstitialis) or Russian knapweed (Centaurea repens). The toxicity results in necrosis of the substantia nigra and globus pallidus (i.e. nigropallidal encephalomalacia). Horses must eat the weeds for several weeks. • Weight loss, depression, yawning. • Excessive muscle tone (dystonia) of jaws, resulting in a grinding movement of the jaws without the ability to close the mouth completely. • Inability to prehend food, chew or drink. • Tongue may be drawn into a longitudinal trough. • Fasciculations of affected muscles. Signs stabilize after several days, but the condition is fatal as there is no known treatment. Birdsville horse disease: This disease is caused by toxicity of Indigophera enneaphylla, which contains alkaloids (i.e. indospicine and canavanine) that are arginine antagonists. It occurs in desert areas of Australia in the spring and summer. • Exaggerated hackney-type action in front. • Reduced flexion with toe-dragging behind. • Head and tail are held high. • May gallop frantically on the spot, eventually the hind limbs spreading and sinking as the animal becomes recumbent. In mild cases, complete recovery can occur but toe-dragging may persist. The toxicity can be prevented by feeding a diet rich in arginine such as alfalfa or peanut meal. Fluphenazine, a potent phenothiazine, is used in humans for its antipsychotic effects and has been used in horses for its sedative effects. Phenothiazines block dopamine, which is the cause of the significant extrapyramidal side-effects reported after use of fluphenazine. Clinical signs include depression, profuse sweating, pawing, striking, agitation, circling, hypermetria, and refusal to walk. Horses may respond to diphenhydramine hydrochloride; however, horses may seriously injure themselves during the course of this toxicosis (Figure 11.5).
Neurology
11.1 Diagnostic approach to neurological diseases
Physical examination
Ancillary diagnostic tests
The brain
Hydrocephalus
Perinatal asphyxia syndrome/hypoxic ischaemic encephalopathy/neonatal encephalopathy/neonatal maladjustment syndrome (dummies, barkers, wanderers)
Congenital cerebellar disease
11.3 Trauma
Vestibular syndrome
Treatment of traumatic brain injury
11.4 Toxic conditions
Plant poisons
Fluphenazine toxicity
11.5 Infectious conditions
Togaviral equine encephalitides: Alphavirus and Flavivirus species