Chapter 11 Neurological Examination and Neurological Conditions Causing Gait Deficits
Diagnosis
Hematology and Serology
In most horses serum chemistry screens and hematological tests are not particularly helpful; however, in horses with a gait deficit caused by an underlying muscle disease, evaluation of aspartate transaminase (AST) and CK levels may be helpful. Stage of training, exercise pattern, and whether the blood specimen was obtained after exercise preceded by a day of rest must be considered in evaluation of enzyme levels. If a horse consistently has abnormally elevated enzyme levels, then the horse has rhabdomyolysis, and the clinician must decide whether the condition is causing or contributing to the horse’s abnormal gait. Plasma CK and AST levels do not increase simply because of muscle atrophy; rhabdomyolysis must occur to increase the enzyme levels in the blood (see Chapter 83). Elevated plasma concentrations of CK and AST in horses that are not being exercised suggest a primary muscle disorder, such as (but not limited to) polysaccharide storage myopathy (see Chapter 83). An elevation in white blood cell count and fibrinogen level indicates inflammation. In our experience, elevation in fibrinogen level is a more consistent indicator of inflammation in the adult horse than is elevation in white blood cell count.
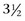 -inch), 18- or 20-gauge spinal needle is directed toward the horse’s lower lip with the head held in a flexed position. It is important that the needle remain on the midline as it is advanced, because otherwise it will be too far lateral to enter the subarachnoid space. The needle is initially inserted to a depth of approximately 2.5 cm (1 inch) and then gradually advanced. While the needle is gradually advanced to the subarachnoid space, it should be held carefully to prevent penetrating the spinal cord when advancing through the atlantooccipital membrane and the dura mater. Usually a “pop” is felt as the needle advances through the dura; however, this finding is not consistent and the stylette should be frequently removed to observe for flow of CSF. CSF usually flows from the needle once the subarachnoid space is entered; however, once a substantial depth has been reached (about 5 to 8 cm [2 to 3 inches] in an average-size horse), some clinicians advise gentle and frequent aspiration with a small syringe.
-inch), 18- or 20-gauge spinal needle is directed toward the horse’s lower lip with the head held in a flexed position. It is important that the needle remain on the midline as it is advanced, because otherwise it will be too far lateral to enter the subarachnoid space. The needle is initially inserted to a depth of approximately 2.5 cm (1 inch) and then gradually advanced. While the needle is gradually advanced to the subarachnoid space, it should be held carefully to prevent penetrating the spinal cord when advancing through the atlantooccipital membrane and the dura mater. Usually a “pop” is felt as the needle advances through the dura; however, this finding is not consistent and the stylette should be frequently removed to observe for flow of CSF. CSF usually flows from the needle once the subarachnoid space is entered; however, once a substantial depth has been reached (about 5 to 8 cm [2 to 3 inches] in an average-size horse), some clinicians advise gentle and frequent aspiration with a small syringe.


