Chapter 19 Neurologic and Musculoskeletal Diseases
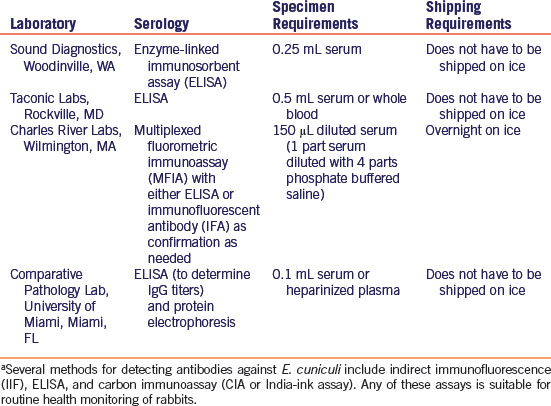
Signs of neurologic disease in rabbits include behavioral changes, head tilt (labyrinthine torticollis or wry neck), nystagmus, tremors, paresis, paralysis, and seizures. Head tilt, usually an indication of vestibular dysfunction, can be central (cerebellum, brainstem) or peripheral (inner ear) and was the most common clinical sign noted in a retrospective study of rabbits with neurologic disease (Table 19-1).22 Ataxia, paresis, and paralysis can also be caused by central (brain or spinal cord) or peripheral nerve disease. Subtle or overt behavioral changes, such as hyperesthesia, may be caused by central or peripheral disease, while seizures and rolling indicate brain lesions. Differentiation of the causes of encephalomyelitis is challenging, and sometimes more than one cause is present in the same animal.22,24 Along with signalment and history, it is essential to perform a thorough neurologic examination to localize the underlying lesion and determine whether the disease is local or multifocal/diffuse. The neurologic examination will help the clinician form a differential diagnosis list and aid in directing appropriate diagnostic procedures. Detailed information on rabbit neuroanatomy, performing a rabbit neurologic examination, and neurodiagnostics has been previously published.48
Table 19-1 Etiology and Clinical Signs in a Retrospective Study of 118 Rabbits Presented with Neurological Diseasea
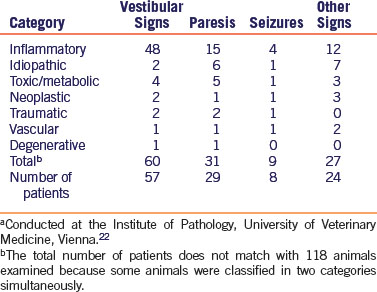
Diagnostic procedures used to help in narrowing the differential diagnoses for neurologic and musculoskeletal disease include clinical pathology (Fig. 19-1), imaging, and serology. Perform a complete blood count (CBC), serum chemistries, and urinalysis to help identify systemic diseases that may manifest themselves with neurologic signs. Take radiographs in cases of lameness, gait abnormalities, head tilt, and spinal abnormalities. Magnetic resonance imaging (Fig. 19-2) is useful in looking for fine detail, including central nervous system soft tissue, and computed tomography is preferred for evaluation of the inner ear, skull, and vertebral column. The determination of antibody titers can be useful in looking for infectious etiologies such as encephalitozoonosis, toxoplasmosis, or pasteurellosis.
Parasitic
Encephalitozoonosis
Encephalitzoon cuniculi infection is often associated with neurologic disease in pet rabbits. Encephalitzoonosis seems to be a widespread disease in rabbits, with reports of infection found in 50% to 75% of conventional rabbit colonies.36 Signs of neurologic disease caused by E. cuniculi include behavioral changes, head tilt, nystagmus, ataxia, rolling, or seizures and often follow a stressful event in the rabbit’s life. E. cuniculi has shown zoonotic potential especially in immunocompromised humans such as transplant recipients or those infected with human immunodeficiency virus (HIV) as well as children, travelers, contact lens wearers, and the elderly.13 It may, therefore, be important to know the serologic status of many pet rabbits.
E. cuniculi is a microsporidium—an obligate intracellular protozoan parasite. Postnatal transmission often occurs within 6 weeks from an infected dam or contact with other infected animals36; however, vertical transmission is possible.6,12,50 A spore, ingested or inhaled, is the infectious stage of E. cuniculi, with oral ingestion of spores from infected rabbit urine being the most common source of infection. Spores can be found in the urine 1 month after infection and are excreted in large numbers up to 2 months postinfection.23 E. cuniculi spores can survive outside the host for up to 6 weeks at 72°F (22°C). Shedding of spores is essentially terminated by 3 months postinfection. The spore possesses a polar filament that it extrudes into host intestinal mucosal cells, injecting spore contents and initiating infection. Multiplication of the E. cuniculi organism takes place in host alimentary cell vacuoles, with eventual cell rupture and spore invasion of the reticuloendothelial system and systemic circulation by infected macrophages. Initial target organs include those with high blood flow, such as the lungs, liver, and kidney,43 with infection of nervous tissue occurring later in the course of the disease. Further organism multiplication occurs via ordinary fission or schizogony within vacuoles or pseudocysts (schizonts) found in reticuloendothelial cells of target organs. Spores eventually develop and, with time, the pseudocyst becomes overcrowded and ruptures. Cell rupture is associated with a chronic inflammatory response, and most immunocompetent rabbits develop chronic, subclinical infections in a balanced host-parasite relationship associated with granulomatous lesions primarily affecting the brain, kidney, or eyes. A wide range of symptomatology is possible and E. cuniculi has been associated with phacouveitis secondary to lens rupture, myocarditis, vasculitis, and spinal nerve root inflammation in addition to encephalitis, pneumonitis, hepatitis, nephritis, and splenitis.39 Abortion and neonatal deaths have also been attributed to encephalitozoonosis.43
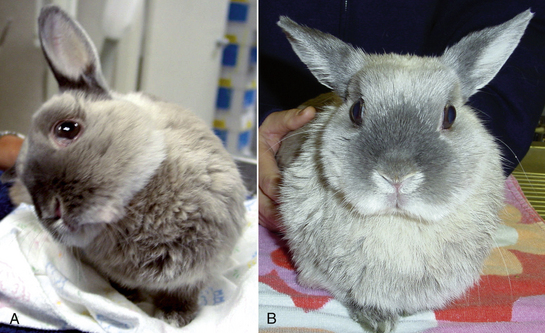
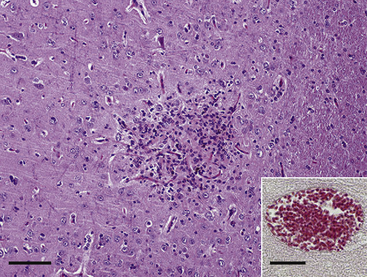
The most commonly recognized neurologic sign in rabbits infected with E. cuniculi is vestibular disease, with infected rabbits showing varying degrees of head tilt, ataxia, circular movements, and nystagmus (Fig. 19-3). Paresis alone is the second most common neurologic sign. In one study determining the extent of histologic lesions in the brain of naturally infected rabbits, the cerebrum was most frequently affected, followed by the leptomeninges (Fig. 19-4).8 The vestibular cores, cerebellum, and spinal cord were less commonly affected in spite of the fact that vestibular disease is the most common clinical sign. In this study, animals were considered infected with E. cuniculi if spores were present; the use of Ziehl-Neelsen stain was slightly more sensitive for spore detection than acid-fast trichome stain. On histopathology, most rabbits showed perivascular inflammatory infiltrates and, less frequently, granulomas. This study found no significant difference for the presence of central nervous system (CNS) granulomas in rabbits with or without neurologic symptoms and concluded that histologic findings of inflammatory lesions are not always indicative of overt encephalitozoonosis as the cause of neurologic signs.
As E. cuniculi organisms spread to various organs, antibodies develop and encapsulation occurs, thus limiting tissue damage and spore excretion. Antibodies become detectable 3 to 4 weeks after infection, with maximum titers occurring 6 to 9 weeks postinfection. A healthy immune system prevents the organisms from multiplying, but the spores remain viable. Immunosuppression, as a result of illness, stress, or aging, may result in overt disease many years after initial infection. Antibodies contribute to resistance by inducing opsonization by macrophages and complement-mediated killing.9
Currently, clinical means of diagnosing definitive antemortem encephalitozoonosis are limited. However, since E. cuniculi infection is persistent, antibodies continue to be produced and, as a general rule, the validity of antibody assays for the detection of E. cuniculi compares favorably with histology in rabbits.20,42,49 Several methods for detecting antibodies against E. cuniculi are available from commercial laboratories in the United States (Table 19-2). A study comparing two indirect immunofluorescence (IIF) assays, two enzyme-linked immunosorbent assays (ELISA), and the carbon immunoassay (CIA, or India-ink assay) for determination of antibodies to E. cuniculi revealed that any of the assays would be suitable for monitoring rabbits for evidence of infection.2 There was no difference in the ability of any of these assays to detect antibodies in serum, although a comparison of techniques showed that IIF, rather than ELISA, is best for quantitative measurement of antibodies. These results, however, may be related to the investigators’ reliance on optical density readings from only one serum dilution.7
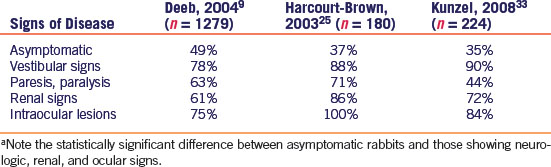
Previous studies profiling the serostatus of rabbits have found a higher incidence of seropositive results in E. cuniculi-suspect rabbits versus clinically normal rabbits2,7,9,25,33 Asymptomatic rabbits have a variable degree of seropositive status, with two studies from the United Kingdom showing 52%31 and 37%25 positive serostatus, one United States study showing 49% seropositivity,9 and an Austrian study33 revealing 35% seropositive results in clinically normal rabbits (Table 19-3). E. cuniculi-suspect rabbits with vestibular signs (head tilt, ataxia, circling, nystagmus, and torticollis) showed the greatest degree of consistent antibody production against E. cuniculi, with 90%,33 78%,9 and 88%25 of rabbits tested being seropositive. Rabbits with other neurologic signs—such as ataxia, paresis, and paralysis—demonstrated 44%,33 63%,9 and 71%25 seroprevalence to E. cuniculi. Rabbits with renal signs (polydipsia, polyuria, perineal scalding, and weight loss plus elevated BUN and creatinine) also demonstrated a significant antibody response with 72%,33 61%,9 and 86%25 being seropositive to E. cuniculi. Finally, rabbits with intraocular lesions (cataracts, phacoclastic uveitis) demonstrated 84%,33 75%,9 and 100%25 seroprevalence to E. cuniculi (Table 19-3).
Table 19-3 Percentage of Rabbits Showing a Seropostive Status to E. cuniculi in Three Separate Studiesa
Other methods advocated for antemortem diagnosis of E. cuniculi include urine antibody analysis and cerebrospinal fluid (CSF) analysis. One study demonstrating an increase in anti-E. cuniculi IgG antibodies in urine samples from asymptomatic seropositive rabbits suggests that the measurement of urine antibodies may be useful in the detection of latent infections in rabbits.17 CSF analysis showed a higher concentration of CSF proteins in rabbits with suspect encephalitozoonosis (based on vestibular disease and/or paresis) compared with normal rabbits.28 Cytologic evaluation of the CSF in these suspect rabbits was characterized by a lymphomonocytic pleocytosis compared with healthy rabbits, which showed low CSF leukocyte counts.28
In human medicine, where E. cuniculi is not an uncommon opportunistic pathogen in immunocompromised patients such as transplant recipients or those infected with human immunodeficiency virus (HIV), polymerase chain reaction (PCR)-based studies have been very successful in identifying E. cuniculi DNA in stool specimens of infected individuals with clinical diarrhea.40 However, studies in rabbits looking for microsporidial DNA to E. cuniculi by PCR in urine samples from those with clinical signs consistent with encephalitozoonosis, significantly high antibody titers to E. cuniculi, and gamma globulinemia on protein electrophoresis did not show statistically significant evidence of the parasite on PCR (C. Cray, personal communication). Another study also found that CSF and urine from rabbits with suspect neurologic and renal encephalitozoonosis, respectively, were negative by PCR for E. cuniculi DNA but did find positive PCR in 4 out of 5 samples of liquefied lens material from rabbits with supposed phacoclastic uveitis.33 These studies confirm that naturally infected rabbits with renal disease or neurologic signs are usually chronically infected, that they either excrete spores for a short period of time postinfection or in low concentrations intermittently, and that PCR-based tests will not prove to be a good antemortem diagnostic tool. In one study involving four rabbits with phacoclastic uveitis, immunohistochemistry was used to identify spores reacting with anti-E. cuniculi antiserum.19 Therefore, in cases of E. cuniculi-induced phacoclastic uveitis where enucleation is performed, histology, PCR analysis, and immunohistochemistry may be used to confirm a diagnosis of antemortem E. cuniculi infection.
In the absence of controlled studies, it is difficult to assess the efficacy of therapeutic agents against E. cuniculi, because latent infections occur and some clinical cases may improve spontaneously without treatment, presumably as a result of the host’s immune response.23 In addition, clinical signs may not be associated with presence of the protozoan itself but rather with the inflammatory response that persists after the organism has been eliminated.25,47
Treatment protocols for rabbits showing clinical signs suspicious for E. cuniculi infection have been based on fundamental principles of therapy for granulomatous inflammation, on studies demonstrating efficacy against human encephalitozoon infections, and on in vitro susceptibility studies of E. cuniculi organisms to various pharmacologic agents. However, there is still no universal agreement on how to effectively treat encephalitozoonosis in rabbits. Several benzimidazole derivatives including albendazole, oxibendazole, and fenbendazole have been used to treat presumptive E. cuniculi infections in rabbits based on their anti-inflammatory actions and their in vitro antiprotozoal activity, including bioenergetic disruptions of membranes and microtubular (tubulin) inhibition of E. cuniculi.15,37 In vitro studies suggest that albendazole can kill the organism and spare the host cell (M. Hawkins, personal communication). In vitro studies have demonstrated that nitazoxide, an antiprotozoal used in equine medicine and advocated on the Internet as an effective treatment option was not effective against the E. cuniculi organism (M. Hawkins, personal communication). Some clinicians advocate the administration of one dose of a short-acting corticosteroid (i.e., dexamethasone, 0.1 mg/kg SC) to control the inflammation associated with the organism when neurologic signs appear acutely. Treatment success of encephalitozoonosis is based on resolution or improvement in clinical signs.
The use of albendazole is partially based on its effectiveness in treating and eliminating Encephalitozoon species from human AIDS patients along with relief of clinical symptoms associated with infection.11 One study demonstrated the in vitro efficacy of fumegillin, thiabendazole, oxibendazole, and albendazole in inhibiting E. cuniculi proliferation in rabbit kidney tissue culture cells.15 Another study showed the efficacy of albendazole in reducing serum creatinine levels in rabbits experimentally infected with E. cuniculi microsporidia (however, the study did not measure creatinine levels in a group of untreated control animals, nor did it do histopathology to prove renal infection with the E. cuniculi organism).5 Some veterinarians have had success with albendazole (30 mg/kg PO q24h for 30 days). Albendazole is embryotoxic and teratogenic in rabbits and has been associated with anecdotal reports of pancytopenia, fever, and death in rabbits15; evaluation of an intratreatment CBC is, therefore, recommended.
Fenbendazole has also been used as a potentially successful chemotherapeutic and prophylactic treatment of rabbit E. cuniculi infections.46 Administration of fenbendazole at 20 mg/kg PO daily for 30 days resulted in the apparent elimination of E. cuniculi spores from the CNS of infected rabbits and has been used with apparent success by the author (PGF) (Fig. 19-1).
Oxibendazole (30 mg/kg PO q24h for 7-14 days, then 15 mg/kg PO q24h for 30-60 days) has been used against rabbit encephalitozoonosis with apparent safety9; however, bone marrow suppression has also been reported anecdotally with oxibendazole; therefore an intratreatment CBC is recommended. In some patients, signs abated during oxibendazole treatment and then recurred after the drug was withdrawn. These patients were then managed on long-term oxibendazole (15-30 mg/kg PO q24h).9
Recent work in mice has shown that a drug designed to block human p38 mitogen-activated protein kinase (MAPK) was successful in treating mice infected with E. cuniculi, suggesting that MAPK inhibitors may represent a novel class of agents with a broad spectrum of antiparasitic activity that may be applied to infection with the Encephalitozoon species.51 Additional therapeutic strategies for human microsporidial infections may someday be applied in the domestic rabbit; these include agents that target the microsporidial polyamines (e.g., polyamine analogs), methionine aminopeptidase 2 (e.g., fumagillin-related compounds), chitin inhibitors (e.g., nikkomycins), topoisomerases (e.g., fluoroquinolones), and tubulin (e.g., benzimidazole-related compounds).13
Control of encephalitozoonosis may be most effective in rabbitries, where transmission usually occurs. The acute phase of the disease usually occurs in very young rabbits, and spores are shed in urine until antibodies are produced. A simple test to determine whether rabbits are shedding spores in the urine is needed to clarify potential transmission duration time. This information could then guide recommendations of quarantine periods when new rabbits are introduced. It may then be possible to select E. cuniculi antibody-negative breeders and to develop encephalitozoon-free rabbit colonies. It must be kept in mind that even if transmission from rabbit to rabbit is prevented, the possibility exists of transmission of spores from exposure to rodents, including mice, hamsters, and guinea pigs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree