CHAPTER 16 Neoplasms, Cysts, Hamartomas, and Keratoses
Cutaneous oncology
Unlike in other domestic animal species, the risk for cutaneous neoplasia in horses generally does not increase with age.8 Saddle horses (a composite of cross-breeds) are at increased risk for cutaneous neoplasia.8 Male horses appear to be predisposed to develop mast cell tumors.8 Breed predilections for cutaneous tumors are presented in Table 16-1.
TABLE 16-1 Breed Predilections for Cutaneous Neoplasms and Nonneoplastic Tumors
| Squamous cell carcinoma | American Paint, Appaloosa, Belgian, Clydesdale, Palomino, Pinto, Shire |
| Sarcoid | Appaloosa, Arabian, Quarter Horse |
| Hemangioma | Arabian |
| Melanoma | Arabian, Lipizzaner, Percheron |
| Dermoid cyst | Thoroughbred |
| Linear epidermal hamartoma | Belgian |
| Actinic keratosis | American Paint, Appaloosa, Belgian, Clydesdale, Pinto, Shire |
Numerous surveys of skin tumors in horses have been published.8 The skin is the most common site of neoplasia in the horse, accounting for about 50% of all equine neoplasms,2,4,6,8 and most equine cutaneous neoplasms are mesenchymal in origin and biologically benign. The most common cutaneous neoplasms in the horse as reported in the veterinary literature are sarcoids, squamous cell carcinomas, papillomas, and melanocytoma/melanoma. In two retrospective studies of skin-biopsy specimens submitted to veterinary diagnostic laboratories in the United States, the most common equine cutaneous neoplasms were sarcoid, melanoma, papilloma, squamous cell carcinoma, and mast cell tumor (Table 16-2).8,10 Skin neoplasms accounted for 37.5% of the equine skin biopsies and 8.8% of the total equine biopsies submitted.
TABLE 16-2 Summary of Cutaneous Neoplasms as Cited in Two Retrospective Studies
| Neoplasm | Northeast United States8 | Northwest United States10 |
|---|---|---|
| Sarcoid | 35.3 | 51.4 |
| Melanoma | 13.9 | 5.4 |
| Papilloma | 10.5 | 4.3 |
| Squamous cell carcinoma | 6.9 | 18.3 |
| Mast cell tumor | 6.9 | 3.4 |
| Fibroma | 6.2 | 1.5 |
| Melanocytoma | 4.8 | 4.1 |
| Basal cell tumor | 2.8 | 0.4 |
| Hemangioma | 2.3 | 0.6 |
| Lymphoma | 2.2 | 2.1 |
| Fibrosarcoma | 2.1 | 1.9 |
| Schwannoma | 2.0 | 1.1 |
| Undifferentiated sarcoma | 1.1 | – |
| Epitrichial sweat gland adenoma | 1.0 | 0.2 |
| Lipoma | 0.4 | 0.7 |
| Myxoma | 0.2 | – |
| Hemangiosarcoma | 0.2 | 0.7 |
| Malignant fibrous histiocytoma | 0.2 | 1.3 |
| Undifferentiated carcinoma | 0.2 | – |
| Epitrichial sweat gland carcinoma | 0.1 | – |
| Lymphangioma | 0.1 | – |
| Leiomyoma | 0.1 | – |
| Carcinosarcoma | 0.1 | – |
There were 725 and 536 total neoplasms in Refs. 8 and 10, respectively.
The key to appropriate management and accurate prognosis of cutaneous neoplasms is specific diagnosis. This can be achieved only by biopsy and histologic evaluation. Exfoliative cytologic techniques (aspiration and impression smear) are easy and rapid and often provide valuable information about neoplastic cell type and differentiation. However, exfoliative cytologic evaluation is inferior to and is no substitute for biopsy and histopathologic examination. Historical and clinical considerations often allow the experienced clinician to formulate an inclusive differential diagnosis on a cutaneous neoplasm, but variability renders such “odds playing” unreliable. In short, “a lump is a lump” until it is evaluated histologically. The detailed histopathologic description of equine cutaneous neoplasms is beyond the scope of this chapter. Only the histopathologic essence of individual neoplasms is presented here. The use of markers—enzyme histochemical and immunohistochemical methods for identifying specific cell types—has increased and has facilitated the diagnosis of neoplastic conditions. Examples of these markers are presented in Chapter 2.
Clinical management of cutaneous neoplasms may include surgery, cryosurgery, electrosurgery, laser surgery, radiotherapy, chemotherapy, immunotherapy, radiofrequency hyperthermia, phototherapy, and combinations of these.1 Brief comments are included under clinical management for each tumor.
Epithelial neoplasms
Papillomas
Cause and pathogenesis
Papillomas are common, benign, viral-induced epithelial neoplasms of the horse.* There are presently three forms of cutaneous viral papillomas recognized in horses. Presumably these three very different clinicopathologic syndromes are caused by different types of equine papilloma viruses (DNA papovaviruses). Viral papillomas are transmitted by direct and indirect (fomite) contact.8 Infection requires damaged skin (e.g., environmental trauma, ectoparasites, ultraviolet light (UVL) damage).
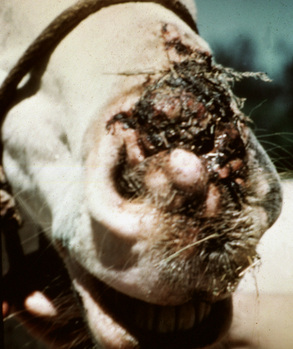
Classical equine viral papillomatosis occurs in young animals, most commonly on the muzzle.8 Experimentally, the incubation period varies from 19 to 67 days.8 Spontaneous remission usually occurs within 2-3 months.8
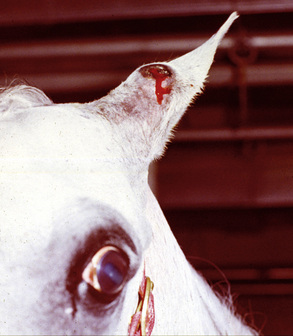
Equine ear papillomas occur in horses of all ages, most commonly on the pinnae, and they rarely, if ever, spontaneously resolve.8 Black flies are probably important in transmitting the causative papillomavirus, both through damaging the skin and as mechanical vectors.
Equine genital papillomas (penis, vulva) occur in older horses (range 13- to 28-years-old), often do not spontaneously resolve, and are probably precursors to the development of some genital squamous cell carcinomas.†
Papillomavirus is fairly stable in the environment and can survive for 63 days at 4-8 °C or for 6 h at 37 °C.8 Humoral immunity (neutralizing antibodies) protects against viral challenge but does not play a role in clearance of established lesions. Cellular immunity is of key importance in papilloma regression. Papillomavirus vaccines (live or formalin-inactivated) are effective preventives, but are of no known therapeutic benefit.
There is great interest and much research in the role of papillomaviruses and oncogenesis.4,33 The viral genome can be divided in parts labeled as L (later region), E (early region), and LCR (long control region). The L1 and L2 genes encode for viral capsid proteins, and the E region consists of genes involved in regulation of viral DNA replication (E1 and E2) or cell proliferation and immortalization (E6 and E7). In addition, E2 protein is an important viral transcription factor regulating expression of E6 and E7 oncogenes. E4 protein binds keratins and facilitates production of virions by disrupting normal cell differentiation. E6 and E7 proteins have oncogene potential and can interfere with cellular factors involved in the control of cell proliferation and the prevention of cell immortalization. E6 and E7 oncogenes are capable of immortalizing cells, inducing cell growth, and promoting chromosomal instability in the host cell. E6 oncoprotein causes degradation of p53 protein by the cellular ubiquitin proteolysis system, leading to unblocking of cell division and host DNA synthesis, which results in chromosomal instability and accumulation of various mutations in affected cells.
Clinical findings
Viral papillomatosis
Viral papillomatosis (warts, verrucae, “grass warts”) occurs in horses younger than 3-years-old and often less than 1-year-old.8 Congenital papillomas have been reported, but these were probably epidermal hamartomas (nevi) (see p. 510). Viral papillomatosis is common in the horse, although some surveys indicate that papillomas account for only 0.6-10.5% of all equine skin neoplasms.8 These surveys are biopsy-based, and since clinicians rarely, if ever, biopsy classical viral papillomas, they clearly underestimate the prevalence of the disorder. There are no apparent breed or sex predilections.
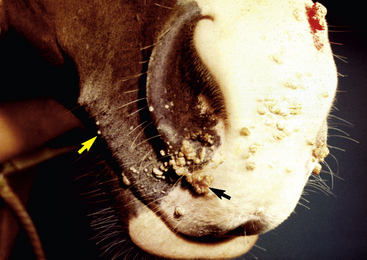
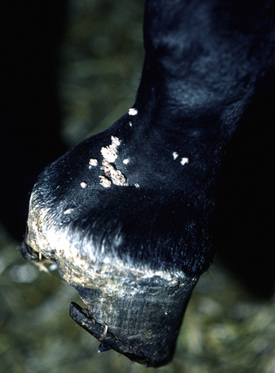
Lesions occur most commonly on the muzzle and lips (Fig. 16-1), less commonly on the eyelid, external genitalia, and distal legs (Fig. 16-2), and rarely elsewhere.8 They begin as small, 1-mm diameter, raised, smooth, shiny, gray to white papules. A rapid growth and increased number of lesions (two to over 100) occurs over a 39- to 54-day period. Fully developed papillomas are 0.2-2 cm in diameter, 0.5 cm in height, broad-based to pedunculated, gray to pink to white in color, and have a hyperkeratotic surface characterized by numerous keratinous, frond-like projections.
Ear papillomas
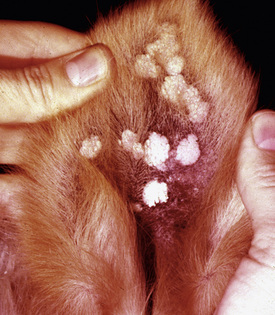
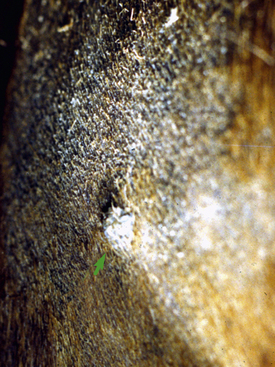
Equine ear papillomas (aural plaque, papillary acanthoma, hyperplastic dermatitis of the ear, “ear fungus”) are common and occur with no apparent breed or sex predilections.8,14 The prevalence of ear papillomas in Mangalargas and Quarter horses in Brazil was 57% and 35%, respectively.15a The condition is seen in horses of all ages, but rarely in animals less than 1-year-old. Lesions begin as small, 1- to 2-mm diameter, well-demarcated, raised, depigmented, shiny papules on the lateral surface of the pinna. The condition is more or less bilaterally symmetric. Lesions enlarge and coalesce to become 1- to 3-cm diameter, white, hyperkeratotic plaques (Fig. 16-3). The surface hyperkeratosis can be scraped off to reveal an underlying shiny, pink, nonulcerated plaque. Similar lesions are occasionally seen around the anus and external genitalia. The condition is asymptomatic but may appear more “active” and symptomatic in summers when the effects of biting flies (especially black flies) complicate matters.
Genital papillomas
Persistent papillomas on the vulva and vagina of a 25-year-old Quarter Horse mare contained papillomavirus antigen and areas of squamous cell carcinoma in situ.15 Other authors have indicated that genital papillomas can undergo malignant transformation to squamous cell carcinoma.3
Diagnosis
Equine papillomas are visually distinctive, and further diagnostic work is rarely indicated. Sarcoid must be considered in the differential diagnosis for any “papilloma” or “wart” in an atypical site in an adult horse.8
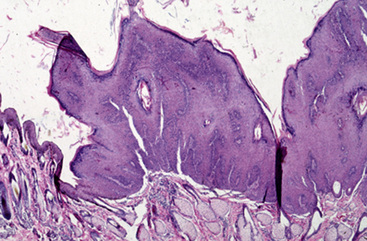
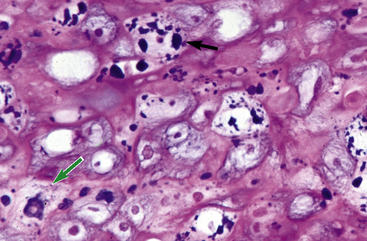
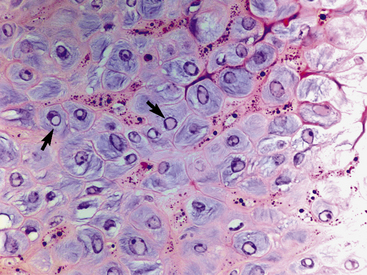
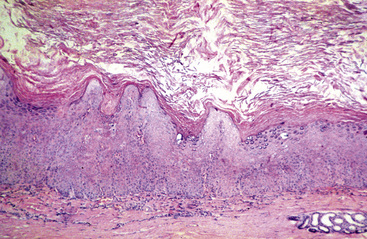
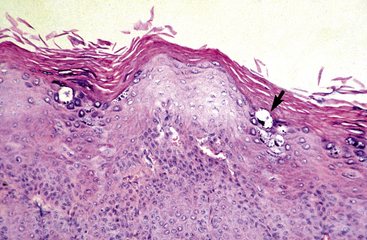
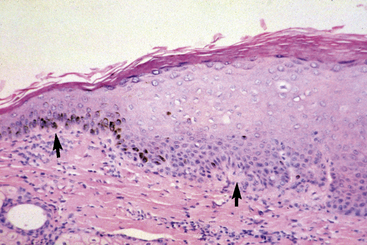
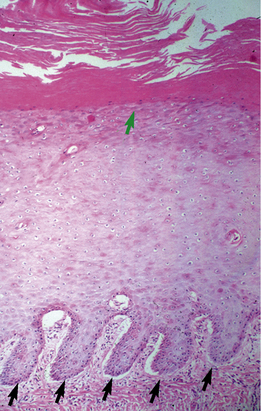
Equine viral papillomatosis has been the subject of extensive pathologic studies.4,8 Histologically, three evolutionary phases are seen: growth, development, and regression. The growth phase is characterized by marked hyperplasia of epidermal basal cells, mild to moderate acanthosis and ortho- and parakeratotic hyperkeratosis, and the presence of very few viral inclusion bodies. The development phase is characterized by the classical features of pronounced papillated epidermal hyperplasia and papillomatosis (Fig. 16-4); koilocytosis (Fig. 16-5); increased numbers, size, and clumping of keratohyalin granules; hypomelanosis; numerous mitoses; and hyperplasia and “nesting” of epidermal basal cells. Viral intranuclear inclusion bodies are visualized in about half of the lesions, most commonly in the stratum corneum, and less commonly in the stratum granulosum and stratum spinosum (Fig. 16-6). Older, regressing lesions are accompanied by increased proliferation of fibroblasts and infiltration of lymphocytes. Electron microscopic examination suggested that the hypomelanosis was due to a disturbance in melanin synthesis and melanocyte-keratinocyte interaction in the epidermal melanin unit. Langerhans cells were reported to decrease significantly in size and number in the development phase, but they markedly increased in number, especially at the dermoepidermal junction, in the regression phase. Cytokeratin expression in viral papillomas was reported to be different from that in normal skin.8
Ear papillomas are characterized histopathologically by changes discussed previously (Figs. 16-7 and 16-8).8 However, the epidermal hyperplasia is only mildly papillated, papillomatosis is mild to absent, and hypomelanosis is striking (Fig. 16-9). These lesions are histopathologically analogous to so-called “verruca plana” or “flat warts” in humans. Electron microscopic studies have revealed intranuclear crystalline arrays of hexagonal viral particles (38-42 nm in diameter), identical to those seen in classical equine viral papillomatosis.
Immunohistochemical studies detect papillomavirus antigen in the lesions of both equine viral papillomatosis and equine ear papilloma.8 Expression of lectin binding in equine viral papillomatosis lesions was the same as that found in normal skin, possibly because of the well-differentiated and organized nature of these neoplasms.8
Clinical management
The lesions of equine viral papillomatosis typically resolve spontaneously within 3 months. Chronically affected animals should be suspected of being immunosuppressed.8 For lesions that must be removed for aesthetic or health reasons, surgical excision or cryosurgery is effective.8 It has been anecdotally stated that surgical excision of some larger lesions may encourage the others to regress. However, a controlled study in horses designed to test this hypothesis showed that the duration of other lesions was not decreased and, in fact, may have been increased.8
Many topical agents have been tried on individual lesions when surgery was impractical.8 Such agents included podophyllin (50% podophyllin; 20% podophyllin in 95% ethyl alcohol; 2% podophyllin in 25% salicylic acid), trifluoroacetic acid, and tincture of benzoin. These agents were applied once daily until remission occurred. All such reports were purely anecdotal. Recent anecdotes include the topical application of imiquimod or bloodroot/zinc chloride-containing products (see Sarcoid). Other treatment anecdotes include the intralesional or intravenous (IV) administration of mycobacterial or Propionibacterium acnes products as immunostimulants and the intralesional administration of cisplatin or interleukin-2 (IL-2).8 It must be remembered that any treatment that provokes an inflammatory reaction may cause permanent depigmentation.
Autogenous tumor cell (“wart”) vaccines have been reported to be “satisfactory” or “of value” for the treatment of equine viral papillomatosis.8 All such reports are purely anecdotal.
Because viral papillomatosis is contagious, spread of disease may be reduced by isolating affected horses and restricting access of immunologically naive horses to infected premises.8 Contaminated stalls, feed and water containers, grooming equipment, and bridlery should be cleaned and disinfected using lye or povidone-iodine compounds.8
Equine ear papillomas rarely, if ever, regress.8 Anecdotal reports have suggested that biopsying or shaving off ear papillomas may result in reduction in size or resolution of lesions, though responses may be temporary (6-12 months).14 Anecdotal reports suggested that the topical application of tretinoin (Retin-A, Ortho; 0.025%, 0.05%, 0.1% cream; 0.01%, 0.025% gel) was effective for the treatment of equine ear papillomas, but others had no success with this form of therapy. Other anecdotal “immunomodulatory” treatments include interferon (IFN) α-2a orally (PO) and/or topically and oral griseofulvin.14
Recent anecdotes include the topical application of imiquimod or Eastern blood root in zinc chloride (see Sarcoid). A recent open-label pilot study evaluated topical imiquimod (Aldara) for the treatment of ear papillomas in 16 horses.16 Imiquimod was applied three times a week, every other week. Side effects in all horses included marked local inflammatory exudation and thick crust formation. Removal of crust before the next treatment was painful and required sedation in most horses. Duration of therapy ranged from 1.5 to 8 months (average 3.3 months). Complete resolution of all lesions was achieved in all horses. With a follow-up period of 12-22 months after treatment for 81% of the horses, there was an 8% recurrence rate.
Squamous Cell Carcinoma
Cause and pathogenesis
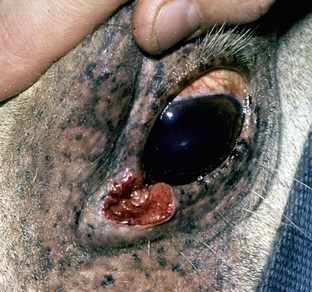
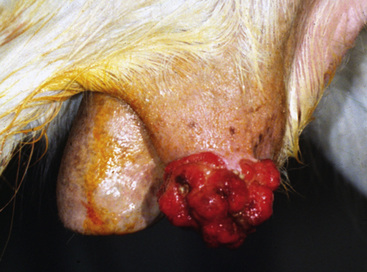
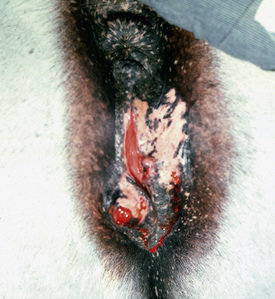
Squamous cell carcinoma is a common malignant neoplasm of the horse arising from keratinocytes.* It is the second most common cutaneous neoplasm of the horse, accounting for 6.9-37% of the equine skin neoplasms in many surveys.2,8,10 It is the most common neoplasm of the equine eyelid and external genitalia.†
Squamous cell carcinoma occurs most frequently in sun-damaged skin and is usually preceded by actinic (solar) keratosis and carcinoma in situ.4,8,17 The prevalence of squamous cell carcinoma increases with increased mean annual solar radiation, increased altitude, increased longitude, decreased latitude, decreased skin and hair pigmentation (white, gray-white, cremello, Palomino), and sparse hair coat.8,17 The p53 gene is overexpressed in equine squamous cell carcinomas to an extent compatible with gene mutation.8 Rarely, squamous cell carcinoma has been reported to arise from burn scars and nonhealing wounds with chronic infection.8,17 In humans, dogs, and cats, papillomaviruses have an etiologic role in some squamous cell carcinomas. Papillomavirus antigen was not detected in equine squamous cell carcinomas.8 However, papillomavirus antigen and in situ squamous cell carcinoma were detected in excised tissue from a horse with multiple vulvar and vaginal papillomas.15 The irritant and carcinogenic properties of equine smegma have been implicated in the etiology of squamous cell carcinoma of the prepuce.8,17
The COX-2 enzyme is thought to aid neoplasm growth and invasion by increasing angiogenesis, invasiveness, and metastasis; inducing resistance to apoptosis; and suppressing immune responses.18,22 COX-2 inhibitors are believed to have antineoplastic activity through the inhibition of PGE2 synthesis. In one study, COX-2 expression was detected in 27% of equine ocular squamous cell carcinomas.22 In another study, COX-2 and COX-1 expression were detected in equine squamous cell carcinomas and in normal equine tissues.18
Clinical findings
The prevalence of equine squamous cell carcinoma increases with age (mean age of 10- to 12-years-old; range of 1- to 29-years-old).2,6,8 Although any breed can be affected, there is a significant increased prevalence in relatively lightly pigmented draft breeds (Belgian, Clydesdale, Shire) and in Appaloosas, American Paints, Pintos, Quarter Horses, and Thoroughbreds that have been chronically exposed to sunlight.8,10,17,21 Sexually intact males and females are significantly less likely (five to two times, respectively) to develop squamous cell carcinoma than castrated males.8,17,21
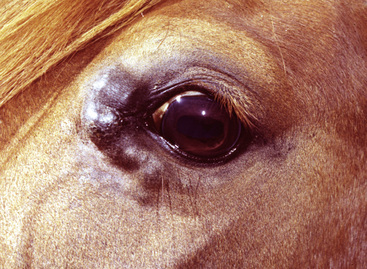
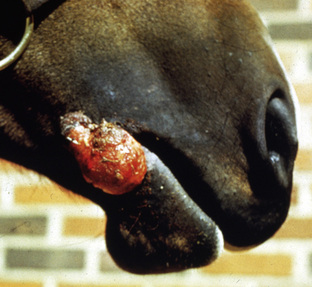
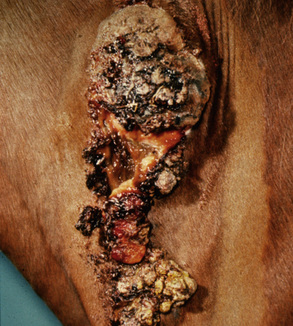
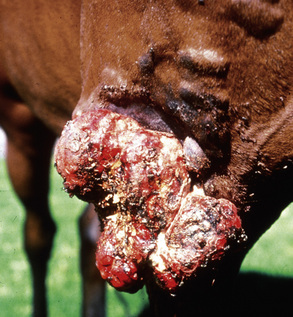
Squamous cell carcinoma can occur anywhere on the body, especially at mucocutaneous junctions.4,8,10 The most commonly affected areas are the eyelid (Figs. 16-10 and 16-11), prepuce (Fig. 16-12), and vulva (Fig. 16-13). Periocular squamous cell carcinoma may be bilateral in up to 20% of the horses.8,21 Squamous cell carcinomas arising in burn scars and chronically infected, nonhealing wounds become clinically recognizable after 1½ to 8 years.8 Lesions are usually solitary, poorly circumscribed, 0.5-6 cm in diameter, beginning as nonhealing, enlarging, granulating ulcers (Fig. 16-14) or as proliferative, often cauliflowerlike masses.8,19 The lesions may be painful. Necrosis and a foul odor are common. In the early stages, persistent, nonhealing, ulcerative lesions appear similar to granulation tissue and may be mistaken for delayed wound healing. Eventually these granulating, ulcerative lesions develop a craterlike appearance with indurated borders. Productive squamous cell carcinomas resemble a papilloma, but with a broader base (cauliflowerlike). Precursor lesions (see Actinic Keratosis) are often not observed by owners. Inflammation and secondary infection (bacteria, Habronema spp., fungi [Pythium, Basidiobolus]) may be complicating factors. Hypercalcemia and pseudohyperparathyroidism were reported in a mare with metastatic vulvar squamous cell carcinoma.8
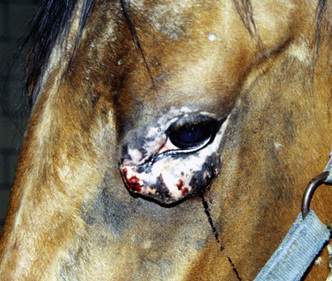
Figure 16-11 Squamous cell carcinoma. Proliferative, ulcerated, depigmented mass involving lower eyelid and medial canthus.
Diagnosis
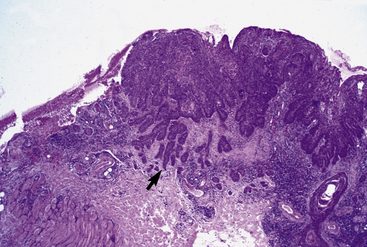
The differential diagnosis of squamous cell carcinoma includes numerous neoplastic and granulomatous disorders. Cytologic examination may be useful for establishing a presumptive diagnosis, showing atypical keratinocytes. Histologically, squamous cell carcinoma consists of irregular masses or cords of keratinocytes that proliferate downward and invade the dermis (Fig. 16-15).4,8 Frequent findings include keratin formation, horn pearls, intercellular bridges, mitoses, and atypia. Solar elastosis may occasionally be seen. Squamous cell carcinomas are positive for cytokeratin, and such examinations may be critical in establishing the true identity of spindle cell and clear cell varieties.
An intense inflammatory response consisting of numerous CD3+ T-lymphocytes, CD79+ B-lymphocytes, immunoglobulin G+ plasma cells, and macrophages is associated with equine squamous cell carcinomas.8 This response was not correlated with the histologic grade or invasiveness, suggesting that the local antitumor immune response failed to prevent tumor invasion or metastasis.
Clinical management
Squamous cell carcinomas are generally locally invasive but slow to metastasize.6,8,19 Up to 20% of the lesions will eventually show metastasis to local lymph nodes and, less commonly, to the lungs.2,8 Poorly differentiated lesions are more likely to metastasize and have an unsuccessful therapeutic outcome.23 Treatment may include surgical excision, cryosurgery, radiofrequency hyperthermia, laser surgery, radiotherapy, chemotherapy, immunotherapy, or combinations of these.1,2,6,8 Treatment is most successful when initiated early in the course of the disease and when surgical excision is used in conjunction with other adjunctive therapies.
Treatment of eyelid squamous cell carcinoma depends on the size of the lesion, location of the lesion, success or failure of prior therapy, economics, and availability of therapeutic modalities to the veterinarian or referral center.8 The prognosis is better for small lesions (less than 1 cm diameter), and preservation of the functional integrity of the eyelid and eyelid margin is critical. Treatments that preserve as much normal tissue as possible are most appropriate, and surgical excision alone is seldom indicated.
Small lesions (less than 1 cm diameter and less than 0.2 cm depth) can be managed successfully by cryosurgery, radiofrequency hyperthermia, CO2 laser ablation, or beta radiation.1,8 Lesions 1-2 cm in diameter and greater than 0.2 cm in depth can usually be treated by cryosurgery, laser therapy, interstitial radiotherapy, or intratumoral cisplatin injections. Large lesions (greater than 2 cm in diameter and greater than 0.2 cm in depth) require careful consideration of therapeutic options, availability of various options, and a guarded long-term prognosis.
Surgery, cryosurgery, radiofrequency hyperthermia, laser surgery
Wide surgical excision, where feasible, is the treatment of choice.1,2,6,8 In periocular squamous cell carcinoma, the recurrence rate is 30-44%. The median survival time for periocular squamous cell carcinoma was 47 months, with 22% of the horses having died or been euthanized due to their disease. Survival time is inversely related to tumor size. Surgical excision of preputial squamous cell carcinoma is unsuccessful in 33-44% of cases.8,23
In a small number of cases treated with CO2 laser ablation, the recurrence rate was about 22%.1,8 Cryosurgery may be effective in 67-87% of the horses treated and is most effective for lesions 2 cm and smaller in diameter.1,2,6,8 Surgical debulking (cytoreduction) should precede the cryosurgical treatment of larger lesions. Leukotrichia and leukoderma should be anticipated. Radiofrequency hyperthermia may be effective in up to 75% of the small superficial lesions.1,8 Leukotrichia and leukoderma should be anticipated.
Radiotherapy
Radiotherapy, where available and feasible, can be very useful. Teletherapy or external beam radiotherapy (orthovoltage or megavoltage) may be effective in 75-100% of the horses treated.1,6,8 Beta-radiotherapy by surface brachytherapy (plesiotherapy) from strontium 90 may achieve a 2-year cure rate of 89%, but is only effective for lesions that are less than 0.2 cm in diameter and less than 4 mm in depth. Gamma-radiotherapy by interstitial brachytherapy (using various implants or “seeds” such as gold 198, iridium 192, cobalt 60, cesium 137, or radon 222) may achieve a 2-year cure rate of 70-100%.8 The recurrence rate of ocular and adnexal squamous cell carcinoma was significantly lower when surgery was combined with adjuvant radiation therapy.21 Complications of radiotherapy include local necrosis, alopecia, and depigmentation; damage to normal structures; corneal opacity; and cataracts. Disadvantages of radiotherapy include extreme expense, limited availability, risks associated with human exposure, prolonged biologic isolation of the patient, and licensing restrictions for their handling. Each state and country has specific radiation hazard laws that must be satisfied.
Chemotherapy
Cisplatin in medical grade sesame seed oil (see Sarcoid) was injected intratumorally (1 mg/cm3 of tissue) through a 22- to 25-gauge needle, four times at 2-week intervals with or without prior cytoreductive surgery, with a mean relapse-free interval of 41 months and an overall relapse-free survival rate of 92% at 1 year and 77% at 4 years.8,9 An overall cure rate (defined as local tumor control at 4 years) of 88% was achieved.9 Treatments were well-tolerated, with local reactions most likely to occur and be more severe after the third or fourth treatments. All treatments were performed with sedation. Cisplatin is a broad-spectrum antineoplastic agent that binds to DNA, causing interstrand and intrastrand cross-links, and procedures for proper handling and disposal must be followed.2,7,9
5-Fluorouracil (Efudex, Roche Derm) was applied topically as a 5% cream to squamous cell carcinomas of the external genitalia with or without prior cytoreductive surgery.8 For males, 5-fluorouracil was applied every 14 days for two to seven treatments (mean five). In females, the agent was applied daily for 1-8 months (mean 4). Complete remissions were achieved in all cases, with no recurrences after follow-up periods of 5-52 months. Side effects were not observed. Three horses with superficial, ulcerative squamous cell carcinoma involving the external nares, lip, or muzzle were treated daily for 30 days with 5-fluorouracil cream.8 Erythema, swelling, blistering, and ulceration occurred during the treatment period, and two of the three horses were still in remission 1 year later. 5-fluorouracil is a fluorinated pyrimidine that blocks the methylation reaction of deoxyuridylic acid to thymidylic acid, thus interfering with the synthesis of DNA. Adverse reactions to topically administered 5-fluorouracil in humans include pain, pruritus, burning, and hyperpigmentation. Thus, caution is warranted when applying this agent (e.g., wear disposable gloves, avoid applying to normal skin). Intratumoral administration of 5-fluorouracil was not effective.8
Intratumoral injections of bleomycin are effective.8 A comparison of intratumoral administration of cisplatin versus bleomycin for treatment of periocular squamous cell carcinomas revealed a 1-year local control rate of 93% for cisplatin and 78% for bleomycin. Treatment with bleomycin is much more expensive.
Piroxicam (Feldene, Roxam) is a nonselective COX inhibitor that is readily available, inexpensive, and easy to administer. Piroxicam (80 mg/day PO) was given to a horse with a squamous cell carcinoma of the lip that had not been cured by intralesional cisplatin, nor by surgical excision followed by doxorubicin, and had metastasized to regional lymph nodes.20 Complete remission and control was maintained for 58 months (piroxicam given every 2-3 days).
Anecdotal reports indicate that bloodroot/zinc chloride-containing products (see Sarcoid) extracts may have some value.8
Immunotherapy
Immunotherapy is of unproven benefit in equine squamous cell carcinoma.2,18 One horse with periocular squamous cell carcinoma and metastasis to the submandibular lymph node was treated with cryotherapy and intratumoral injections of a bacillus Calmette-Guérin (BCG) cell wall emulsion (see Sarcoid). The periocular mass disappeared, the submandibular lymph node returned to normal size, and the horse was normal after a 1½-year follow-up. Other authors have not found BCG to be successful.
Preventive measures include routine cleaning of the prepuce and penis, avoiding exposure to sunlight, if possible, from 9 am to 3 pm (maximum UVL intensity is from 11 am to 2 pm), and UVL-protective blankets and fly masks.8,17 The development of new squamous cell carcinomas is common when UVL is not avoided. In breeds with high risk of ocular squamous cell carcinoma, tattooing is permitted by some organizations.17
Basal Cell Tumor
Cause and pathogenesis
The term basal cell tumor has been used in veterinary literature to classify a large group of neoplasms of domestic animals. Numerous histopathologic “subclassifications” have been applied to these neoplasms: medusa head, garland or ribbon, trabecular, solid, cystic, adenoid, basosquamous, and granular cell.4,8 In general, these various histopathologic types are often found within the same neoplasm and apparently offer no useful clinical, prognostic, or therapeutic information. Most veterinary basal cell tumors are benign and not contiguous with the basal cell layer of the epidermis; these lesions generally show differentiation toward follicular structures and have been reclassified in dogs and cats.4 It is likely that such reclassification would be appropriate in horses as well. However, because the number of basal cell tumors carefully described and illustrated in equine skin is very small, the “basal cell” terminology will be retained for the present.
These are rare, benign neoplasms of the horse that are thought to arise from the basal cells of the epidermis. Although these tumors accounted for 2.8-5.7% of the equine cutaneous neoplasms in some surveys, they were only briefly mentioned in other surveys and not even acknowledged in most.8,10 The cause of basal cell tumors in horses is unknown. In humans, there is a strong correlation between exposure to UVL and the development of basal cell tumors. However, the occurrence of these lesions in haired, dark-skinned areas of horses indicates that UVL exposure is of no etiologic importance in this species.
Clinical findings
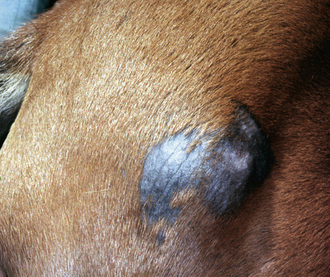
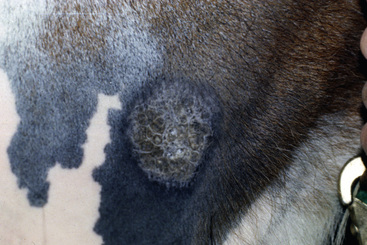
Basal cell tumors occur in horses with no apparent breed or sex predilections. The mean age of affected horses is 10.6-years-old (range 4- to 26-years-old).8 The lesions are solitary, rounded, firm, well-circumscribed, and 0.5-5 cm in diameter. The overlying skin may be normal, hyperpigmented, alopecic, or ulcerated. The lesions can occur anywhere, but are most commonly reported on the distal limbs, trunk, face, and neck (Fig. 16-16).8,10,12
Trichoepithelioma
Cause and pathogenesis
Trichoepitheliomas are benign neoplasms thought to arise from keratinocytes that differentiate toward all three segments of the hair follicle.4,8 They are positive for cytokeratin. The cause of trichoepitheliomas is unknown. In humans, a syndrome of multiple trichoepitheliomas is hereditary.
Clinical findings
Trichoepitheliomas are extremely rare in the horse. A 22-year-old Thoroughbred gelding was presented for a solitary 4-cm diameter, hyperkeratotic, nodular trichoepithelioma over the mandible.8
Diagnosis
Histopathologically, trichoepitheliomas vary considerably, depending on the degree of differentiation and whether the tumor is primarily related to the follicular sheath or the hair matrix.4,8 Frequent characteristics include horn cysts, differentiation toward hair folliclelike structures, and formation of abortive or rudimentary hairs (Fig. 16-17).
Dilated Pore
Cause and pathogenesis
The dilated pore (dilated pore of Winer) is extremely rare in horses.8 The cause of this lesion is unknown, although evidence favors a developmental origin arising from the combined forces of obstruction and intrafollicular pressure leading to hair follicle hyperplasia.
Clinical findings
A single case has been described in the horse.8 The lesion was firm, well-circumscribed, and located caudal to the point of the elbow. Surgical excision was curative.
Diagnosis
Histopathologically, the lesion is characterized by a markedly dilated, keratinized, pilar infundibulum lined by an epithelium that is atrophic near the ostium but increasingly hyperplastic toward the base (Fig. 16-18). The epithelium at the base shows psoriasiform hyperplasia with rete ridges and irregular projections into the surrounding dermis.
Sebaceous Gland Tumors
Cause and pathogenesis
Sebaceous gland tumors are very rare skin neoplasms of the horse arising from sebocytes.8 Their cause is unknown.
Clinical findings
There is little clinicopathologic information recorded on these neoplasms, since they are typically listed only as “sebaceous adenoma” or “sebaceous gland tumor” in surveys of equine skin tumors.8 A sebaceous carcinoma of the prepuce that metastasized to the peritoneal cavity was reported in one horse.
Diagnosis
Cytologic examination reveals clustering of lipidized sebocytes and a variable percentage of basaloid cells. Histopathologically, sebaceous gland tumors are classified as nodular sebaceous hyperplasia (greatly enlarged sebaceous glands composed of numerous lobules grouped symmetrically around centrally located sebaceous ducts), sebaceous adenoma (lobules of sebaceous cells of irregular shape and size, which are asymmetrically arranged and well-demarcated from the surrounding tissue and contain mostly mature sebocytes and fewer undifferentiated germinative cells), sebaceous epithelioma (tumor similar to basal cell tumor but containing mostly undifferentiated germinative cells and fewer mature sebocytes), and sebaceous carcinoma (tumor with pleomorphism and atypia).4,8 Sebaceous gland tumors are positive for cytokeratin.
Sweat Gland Tumors
Cause and pathogenesis
Epitrichial sweat gland tumors are rare neoplasms of the horse arising from the glandular or ductular components of epitrichial sweat glands. They accounted for only 0.2-1.1% of the cutaneous neoplasms in two surveys.8,10 The cause of these tumors is unknown.
Clinical findings
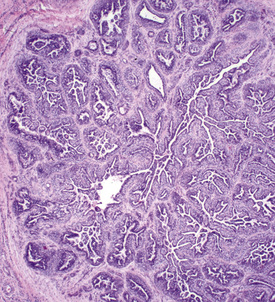
These neoplasms are most commonly benign and most commonly reported on the pinna and vulva (Fig. 16-19).8 Lesions are usually solitary, firm to cystic, well-circumscribed nodules. The overlying skin may be normal, alopecic, or ulcerated. One horse developed multiple epitrichial sweat gland carcinomas on the distal aspect of all four legs over 2 years.24 The lesions were well-circumscribed, firm, and up to 2 cm diameter, and became alopecic with a smooth surface. The horse remained healthy with no evidence of metastases.
Diagnosis
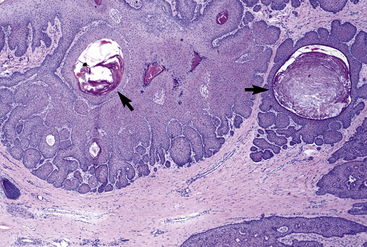
Cytologic examination may reveal clusters of epithelial cells containing secretory droplets. Ulcerated lesions may mimic squamous cell carcinomas. Histopathologically, epitrichial sweat gland tumors are characterized by benign or malignant proliferations of epitrichial sweat gland epithelium, which may feature predominantly secretory or ductal epithelium and may be solid or cystic in appearance (Fig. 16-20).4,8,24 Sweat gland tumors are positive for cytokeratin.
Clinical management
Clinical management of epitrichial sweat gland tumors may include surgical excision, cryosurgery, or observation without treatment. The horse with multiple epitrichial sweat gland carcinomas had several lesions successfully treated by the topical application of imiquimod (see Sarcoid).24
Mesenchymal neoplasms
Tumors of Fibroblast Origin
Due to the early confusion and controversy over the histologic diagnosis of the equine sarcoid, many of the surveys on equine cutaneous neoplasia published prior to 1980 are misleading.8 Most of these publications indicated that “fibropapillomas,” “fibrosarcomas,” “fibromas,” or “invasive fibromas” were the most common equine cutaneous neoplasm. In most instances, the clinical information provided for these horses was typical for that of sarcoids. The histologic variability of the equine sarcoid is discussed later.
Sarcoid
Cause and pathogenesis
The sarcoid is a common, locally aggressive, typically nonregressing, fibroblastic cutaneous neoplasm of the horse.8,14,30,43 It is the most common skin neoplasm of the horse, accounting for 35.3-90% of the total in numerous surveys.* Sarcoids accounted for 0.7-2% of the equine clinical cases presented to two American and two Swiss university clinics.8 These tumors adversely affect the material value of horses, and they often compromise the use of the animal because of their location, although they are not life-threatening in most instances. In the United Kingdom, the equine sarcoid is probably the most common cutaneous reason for euthanasia, and the loss to the equine industry is considerable.8
Sarcoids frequently occur in areas subjected to trauma (wounds, tack, insects), and there may be a history of a previous wound 3-6 months earlier.† The lesions may be spread to other areas on the same horse or possibly to other horses through biting, rubbing, fomites, and insects. Epizootics in herds and examples of multiple-case horse families living together are consistent with an infectious origin. Circumstantial evidence incriminates flies in the pathogenesis and epidemiology of sarcoids.8 Bovine papillomavirus (BPV) DNA sequences have been found in flies, and the same viral DNA sequences were detected in the horses from which the flies were removed.33,33a Sarcoids have been autotransplanted, but transmission to other sites on donor horses or to other horses by injection of various sarcoid tissue extracts has not been routinely successful.8
It is widely accepted that BPV types 1 and 2 are associated with the pathogenesis of equine sarcoids.‡ BPV-1 and BPV-2 belong to the genus Δ-papillomavirus, family Papillomaviridae. Molecular techniques have demonstrated BPV-1 and/or BPV-2 DNA sequences in virtually all equine sarcoids.
The papillomavirus genome is transcriptionally divided into a late region and an early region. Early gene products stimulate cellular proliferation and are responsible for the maintenance and replication of the viral genome within the dividing cell.31–33 Three of the BPV early genes, E5, E6, and E7, expressed in equine sarcoids are capable of causing neoplastic transformation of cell cultures. These early gene products downregulate mammalian suppressor gene localization, affect trafficking and modification of cellular proteins, and cause cytoskeleton disruption. The expression of the major transforming BPV proteins E5 and E6 in equine sarcoids implies active papillomavirus infection.32,41
The unique BPV genome variations found in equine sarcoids are also found in equine sarcoid cell lines, and only these variant BPV genomes can transform equine cells.35,40,47,49 These equine cell lines are morphologically transformed, proliferate faster than parental cells, have extended lifespans, and can grow independent of substrate. These characteristics are more marked the higher the levels of viral E5, E6, and E7. Several unique BPV E5 sequences have been detected in equine sarcoids.32 The association, if any, of BPV-1 or BPV-2, of viral genome sequences, or of E5 variants with the type of sarcoid and/or disease course and outcome are still to be determined.
Sarcoids are not associated with a high level of cellular proliferation as assessed by Ki67 expression or the expression of cell cycle regulators such as cyclin-dependent kinase (CDK-2), cyclin A, and P27kip1.8,41 Only a subset of sarcoids exhibit abnormal P53 expression.8,41 These findings suggest that the molecular pathology of sarcoids is generally not associated with abnormal cell cycle control mechanisms.
Although BPV genomes are consistently present in equine sarcoids and early transforming viral proteins are expressed, virions are not produced.8,14,33,47 Contact with virus alone is not sufficient for tumor production, and skin trauma, host immunologic status, and host genetic constitution also play an important role.27,33 Sarcoids do not appear to be induced by an infective cell line.34
BPV DNA sequences and E5/E6 sequences have also been found in normal skin from sarcoid-bearing horses, and skin from normal horses.8,14,26,27 Swabs taken from the normal skin of sarcoid-bearing horses, skin from normal horses living with sarcoid-bearing horses, and the surroundings (stable wall, feed tub) were analyzed for the presence of BPV DNA.26 Normal skin from sarcoid-bearing and in-contact normal horses was positive for BPV DNA in 100% and 44%, respectively, of the animals. BPV DNA was not detected from the surroundings. In another study, swabs and biopsies from the normal skin of sarcoid-bearing horses, skin from normal horses living with sarcoid-bearing horses, skin from normal horses living with papilloma-bearing cattle, and skin from healthy control horses were analyzed for BPV DNA.27 Samples were positive in 57% of sarcoid-bearing horses, 50% of normal horses living with sarcoid-bearing horses, 73% of normal horses living with papilloma-bearing cattle, and 30% of healthy control horses. Some cases of equine dermatitis contain BPV-1 genomes that express viral transcription genes (E1, E2, and E5).33,48 It would appear that latent infection with BPV is widespread in horses. This may be one explanation for the high rate of recurrence following surgical excision of sarcoids.8,14,31,33
Early studies indicated that BPV DNA sequences were not detected in lymphocyte DNA from sarcoid-bearing horses.8 However, a recent study reported that peripheral blood mononuclear cells (PBMCs) from horses with BPV-1 and/or BPV-2 associated sarcoids contained BPV E5 sequences.28 PBMCs from horses without sarcoids were negative. Thus, it could be that PBMCs may serve as host cells for BPV-1/BPV-2 DNA and contribute to viral latency.
Certain equine leukocyte antigens (ELAs) are associated with susceptibility to sarcoids.8,31,33 ELA alleles A3 and W13 are associated with increased risk in Thoroughbreds and French/Irish/Swiss warmbloods. Alleles B1 and W3 are associated with increased risk in Thoroughbreds. Alleles A1 and A5 are associated with increased risk in Arabians and Freibergers, respectively. Perhaps the low prevalence of the W13 allele in Standardbreds confers genetic resistance and explains their decreased risk compared with Quarter Horses and Thoroughbreds.
Associations between clinical parameters of sarcoids and the ELA system have also been analyzed: A5 was associated with early onset; W13 was associated with increased recurrence rates following surgery; A3 and W13 were associated with increased prevalence.8
Clinical findings
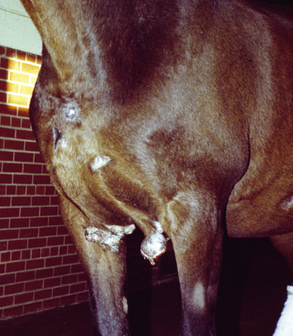
The majority of the veterinary literature indicates that there is no sex predilection, although some studies suggest an increased risk in geldings.8,14 Although any breed can be affected, some studies indicate that Appaloosas, Arabians, and Quarter Horses are at increased risk, while Standardbreds are at decreased risk.2,8,14 Given the etiologic significance of BPVs in equine sarcoids, the increased risk in Appaloosas and quarter horses is interesting, because these are breeds generally found on cattle farms. Although sarcoids may occur in horses of any age, they are extraordinarily rare in horses less than 1-year-old, and the majority of affected animals are 7-years-old and younger.2,8,14,30 Coat color, seasonal, and geographic predilections have not been identified.
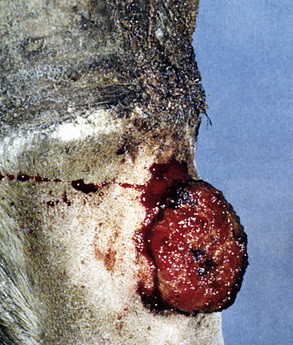
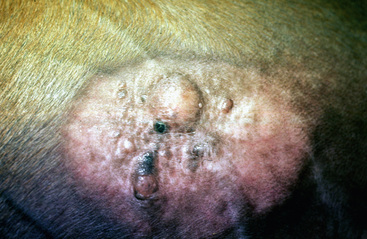
Sarcoids may occur anywhere on the body, but the majority of lesions occur on the head (especially on the pinnae, commissures of the lips, and periocularly), neck, legs, and ventral body surface.* Anywhere from 14% to 84% of affected horses have multiple sarcoids.† Nodular sarcoids, in particular, can have hundreds of masses (0.5-20 cm diameter) in bunches of interconnecting or separate tumors.37 Referral clinics and veterinary schools tend to see animals with multiple lesions, whereas primary care veterinarians often see animals with solitary lesions. The gross appearance of sarcoids can be quite variable, but six broad categories are recognized:‡
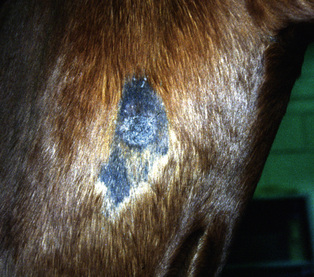
Figure 16-21 Occult sarcoid. Annular, alopecic, hyperkeratotic, slightly raised plaque on neck (area has been clipped).
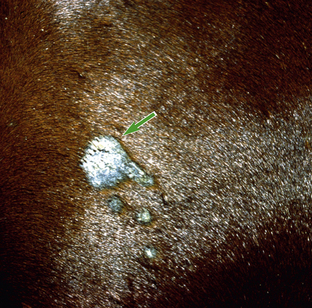
Figure 16-23 Occult sarcoid. Larger original central lesion (arrow) and recent smaller “satellite” lesions on neck.
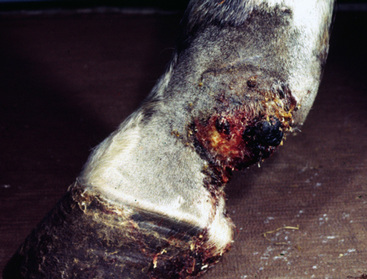
Sarcoids vary in size from 1 cm diameter to enormous (Figs. 16-32 and 16-33). The largest lesions are usually found on the distal limbs, which may reflect that certain sites are more frequently exposed to mechanical irritation that both initiates wounds and stimulates tumor growth.8 All six morphologic forms may have smaller “satellite” lesions at the periphery of the larger primary lesion (Fig. 16-34). Many horses have a range of morphologic forms. Sarcoids generally have a high capacity for local invasion into the surrounding skin and other tissues. This poses a particularly dangerous problem when the eyelid is involved.
Diagnosis
Diagnosis is confirmed by skin biopsy. It is widely accepted that the manipulations used for taking a biopsy or the removal of only part of the neoplasm may stimulate growth of the remaining tissue and include the risk of transforming a quiescent sarcoid into an active proliferating form.* Thus, biopsy of occult, nodular, or small verrucous sarcoids has been discouraged by some clinicians. However, although some sarcoids remain relatively, or even completely, static for years, others multiply on the individual horse, sometimes very rapidly. Even the most benign-looking lesion can explode, often when traumatized but sometimes seemingly spontaneously, into a potentially disastrous mass in a short time.8,14,37 In addition, the variability in clinical morphology of sarcoids and the lengthy differential diagnosis make a clinical diagnosis of sarcoids unreliable. In a study of 345 horses referred for a clinical diagnosis (no biopsy) of sarcoids, 31% were found to have nonneoplastic dermatoses.14 Current thought is that there is generally no contraindication for biopsy of a sarcoid as long as definitive treatment is instituted immediately after the diagnosis is made.2,14,31,37 That said, a biopsy should not be recommended if the owner is not willing to pursue treatment.
Equine sarcoids are characterized histologically by fibroblastic proliferation with associated epidermal hyperplasia and dermoepidermal activity (Fig. 16-35).4,8 However, epidermal hyperplasia, rete ridges, and “picket fence” formation at the dermoepidermal junction may be absent in 46%, 54%, and 52%, respectively, of the cases. These changes are absent more frequently in occult and nodular sarcoids. The dermis shows variable amounts of collagen fibers and fibroblasts in a whorled, tangled, herringbone, crisscross or linear pattern, or combinations of these. Because of this variability of dermal configuration, a given sarcoid or area within a sarcoid could be misdiagnosed as fibroma, fibropapilloma, fibrosarcoma, neurofibroma, neurofibrosarcoma, Schwannoma, or exuberant granulation tissue.8 The Schwannomalike appearance of some areas of some equine sarcoids probably resulted in multiple periocular sarcoids being misdiagnosed as Schwannomas (“neurofibromas”)8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree