CHAPTER 6 Neonatal Septicemia*
Localized and systemic bacterial infections remain a leading cause of morbidity and mortality in the equine neonate despite recent advances in prevention and treatment.1,2 Many factors can influence a foal’s risk for the development of sepsis in the peripartum period. This chapter discusses those factors as well as causative organisms and therapeutic options. In addition, factors influencing prognosis and potential preventive strategies are addressed.
ETIOLOGY
Predisposing Factors and Routes of Infection
Many maternal and postnatal events can predispose an equine neonate to infection, including maternal illness, alterations in gestational length, partial or complete failure of passive transfer of immunity, poor sanitary conditions, and improper umbilical care. Maternal factors predisposing to neonatal sepsis include dystocia, premature placental separation, placentitis, and various other forms of maternal illness (e.g., colic). These problems were contributing factors in 24% of bacteremic foals in a recent study.12 Many of these factors can be interrelated, with placentitis as a primary event and other problems such as premature placental separation occurring secondarily (see Chapter 8). In utero infection of the fetus caused by placentitis occurs typically by ascending infection and often results in premature delivery.13 Because chronic placentitis in the mare often results in precocious fetal maturation, a premature foal born to such a mare likely has a greater chance of being septic but a higher probability of survival than a foal born at a similar gestational age to a mare without placentitis or other chronic stimulation.
Failure of passive transfer of immunoglobulin (FPT) is a major risk factor for equine neonatal sepsis. Because the foal is relatively immunonaive at the time of birth, postnatal transfer of immunoglobulin through ingestion and absorption of colostral antibodies is critical for prevention of foal infection. A number of studies have documented a close relationship between the concentration of foal serum immunoglobulin G (IgG) and incidence of disease.14–17 Clearly, factors other than the magnitude of passive transfer also are involved in determining disease risk. The route and timing of transfer are likely relevant, along with the potential for bacterial challenge. Farm management is particularly important, including general cleanliness, stocking density, exposure to disease, maternal nutrition, and prepartum vaccination and deworming programs. One study has demonstrated that foals with partial FPT were at no greater risk of disease than those with adequate transfer on a well-managed Standardbred farm.18
Postnatal routes of infection include the umbilicus, gastrointestinal (GI) tract, and respiratory tract. Although the umbilicus has been traditionally regarded as an important site for bacterial pathogen entry into the foal, the role of the intestinal tract has been recently reevaluated.19 It is suggested that too much emphasis is placed on the magnitude of colostral antibody transfer, rather than the timing of ingestion of colostrum. This concept was raised 30 years ago, when it was demonstrated that noninvasive Escherichia coli could be absorbed by the intestine of neonatal piglets before gut closure.20 In the pig and the lamb, the ability to absorb macromolecules is regulated by luminal content, and closure can be delayed for up to 5 days by deprivation of milk or colostrum. Conversely, the period before gut closure can be shortened by feeding a large volume of colostrum or milk shortly after birth.21,22
Less work has been performed in the foal, but several key details have been established. The foal does not fully discriminate between maternal IgG and other macromolecules, and absorption of macromolecules occurs through specialized cells by pinocytosis. The absorption of macromolecules peaks shortly after birth and declines to less than 1% by 20 hours.23 Unlike other species, absorption of IgG does not appear to be Fc receptor mediated in the foal. The foal will selectively absorb IgG and immunoglobulin M (IgM) over IgA.24 Unlike the piglet or the lamb, intestinal permeability to IgG cannot be delayed through withholding of macromolecules in the foal.25 It is not known whether premature closure can be induced by the feeding of macromolecules immediately after birth.
Significant postnatal factors other than FPT that affect risk for neonatal sepsis include gestational age and environmental conditions. Foals with exceptionally short or long gestation are at increased risk for the development of sepsis.26 Unsanitary environmental conditions can result in an increased bacterial load to the neonatal GI tract, especially during the initial period of udder seeking.
Causative Organisms
Retrospective studies have examined the most common organisms isolated from blood culture and necropsy specimens of septic foals (Table 6-1). Although gram-positive organisms predominated in the 1940s to 1950, E. coli has been the predominant organism isolated from septic foals in recent studies, regardless of clinic location or methodology.* Era and geographic location appear to play a major role in the significance of other pathogens. In Pennsylvania in the late 1990s, gram-positive bacteria (Enterococcus, Streptococcus, and Staphylococcus spp.) cumulatively played a major role in disease pathogenesis,8 whereas Actinobacillus spp. accounted for approximately 30% of all isolates at Ohio State University in the late 1990s.12 A Georgia study has reported a dramatic decrease in the percentage of E. coli isolates between 1986 and 1990 and later 5-year sampling periods (1991–1995 and 1996–2000).33 The organisms with increased prevalence over the same period were Enterococcus spp. and Staphylococcus spp. A study evaluating trends by decade (1980s and 1990s) in a Florida population found that E. coli remained the predominant isolate, percentages of gram-negative nonenteric and gram-positive organisms remained steady, the percentage of anaerobes increased, and the gram-negative, nonenteric organisms decreased.36
Systemic fungal infections also can occur in neonatal foals. The most frequently isolated organism is Candida albicans, a dimorphic fungus, although other organisms may play a similar role37,38 (see Chapter 53). These infections are typically associated with prolonged hospitalization and invasive monitoring techniques38 or immunodeficiency.37 Prolonged antimicrobial therapy and the administration of parenteral nutrition have been suggested as risk factors for the development of candidiasis. A common clinical sign is fever unresponsive to antimicrobial therapy. Most foals with systemic candidiasis will develop thrush (white plaques on the lingual surface of the tongue) either concurrently or before showing clinical signs of systemic infection; thus a daily oral examination is recommended for all hospitalized foals. Antifungal therapy should be strongly considered in any presumed septic foal that develops thrush and is clearly indicated in any animal with a confirmed isolate.
PATHOGENESIS
Much of the clinical syndrome classically associated with equine neonatal sepsis is caused by a nonspecific inflammatory response to the infectious organism. Many terms have been used to describe this response and its associated syndromes and processes. A set of definitions was described in 1991 by the American College of Chest Physicians and the Society of Critical Care Medicine,3 and a summary of this consensus report follows.
Manifestations of organ dysfunction in the horse can include laminitis and coagulopathy in addition to renal, GI, hepatic cardiovascular, or pulmonary dysfunction.4 The multiple organ dysfunction syndrome (MODS) describes the alteration of organ function in an acutely ill patient such that homeostasis cannot be maintained. MODS can occur either as a primary event (i.e., direct result of trauma) or secondary to a host response. Recently, a syndrome of immunosuppression caused by an exuberant systemic antiinflammatory response resulting in increased circulating levels of antiinflammatory mediators, leukocyte anergy, or increased susceptibility to infection has been termed the compensatory antiinflammatory response syndrome (CARS). If an individual fluctuates between episodes of SIRS and CARS, the term mixed antiinflammatory response syndrome (MARS) applies.5
Endotoxin plays a critical role in the pathogenesis of septic shock in gram-negative sepsis6,7 and is particularly important in the foal, because the most frequently isolated organisms are gram-negative bacteria.2,8 The pathogenesis of sepsis, endotoxemia, and the systemic inflammatory response has been reviewed extensively in both humans and horses and is covered in detail in Chapter 37.3,9–11
CLINICAL FINDINGS
Clinicopathologic Findings
Clinical signs and historical information alone often are sufficient for the clinician to develop a reasonable suspicion of neonatal sepsis. In addition to the physical examination findings, however, laboratory data may be helpful for diagnosis of early sepsis. Leukopenia, characterized by neutropenia, is the most common hematologic finding associated with acute sepsis. In one study, septic foals less than 1 week of age had a lower total white blood cell (WBC) count, both neutrophils and lymphocytes, and higher bands and monocytes than healthy, age-matched controls.27 Premature or dysmature foals also will often have neutropenia in the absence of sepsis; however, septic foals typically have a degenerative left shift and evidence of toxicity (e.g., Döhle bodies, toxic granulation, vacuolization), whereas these findings are not typical of uncomplicated prematurity. In older septic foals (8-14 days) the total WBC count, neutrophils, and bands are higher than in age-matched controls.27 A high fibrinogen concentration at or shortly after birth should raise the suspicion of in utero infection.28
Abnormal serum glucose concentrations are common in septic foals. Hypoglycemia is common initially, especially in foals less than 24 hours of age.28 Although hypoglycemia is related predominantly to decreased intake, endotoxemia can contribute to hypoglycemia by decreasing hepatic gluconeogenesis and increasing peripheral glucose uptake. In the initial phase of treatment, the rate of glucose supplementation should be monitored carefully because many foals develop hyperglycemia in response to dextrose infusion. Other biochemical abnormalities common in septic foals include azotemia and hyperbilirubinemia.2
Common abnormalities found on arterial blood gas (ABG) analysis include acidemia and increased lactate concentrations. One early report demonstrated a high incidence of metabolic, respiratory, or mixed acidosis.28 A recent report has indicated significant differences in arterial lactate concentration between foals with a positive versus negative blood culture, those that met the criteria for SIRS versus those that did not, and those that met the criteria for septic shock versus those that did not.29 These differences were noted at admission and at 18 to 36 hours after admission for the blood culture and SIRS variables, but numbers precluded an analysis for the septic shock variable. Stewart et al.12 have noted that foals with gram-negative enteric bacteremia were more likely to have an elevated arterial carbon dioxide tension (Paco2) than other foals with bacteremia.
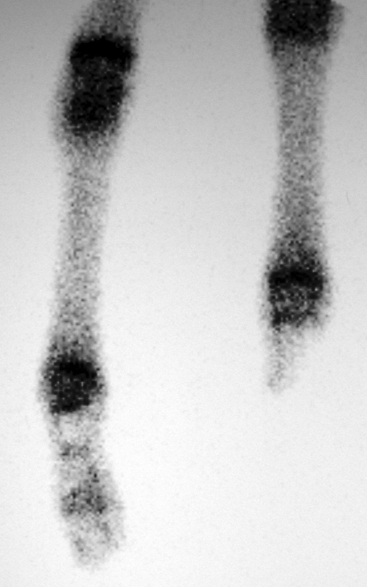
The coagulation and fibrinolytic systems of the septic newborn often are abnormal, with clinically relevant decreases in antithrombin III and increases in prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen and fibrin degradation products (FDPs).27 A detectable plasma endotoxin concentration but not a positive blood culture result was significantly correlated with abnormal PT and APTT in this study; thus endotoxemia, not bacteremia, is likely associated with the development of coagulopathy in septic foals. Some patients may develop active hemorrhage or thrombosis, which can include thrombosis of major arteries, such as the aorta or iliac, femoral, or brachial artery (Fig. 6-1).
Respiratory Involvement
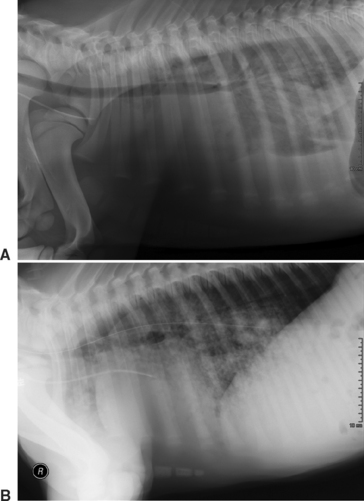
The lungs are a very common site of focal infection in the septic foal, with a reported incidence of pneumonia ranging from 28%12 to 50%.62 Respiratory rate and effort, thoracic auscultation, and rectal temperature often can alert the clinician to the possibility of pneumonia in a patient. Respiratory function is best assessed in septic foals with ABG analysis.2,55 Thoracic radiographs provide an estimation of disease severity and distribution (Fig. 6-2). In addition to hematogenously acquired pneumonia, septic foals are at risk for aspiration of either meconium or milk, depending on their presentation. Directed antimicrobial therapy and the maintenance of an acceptable arterial oxygen tension (Pao2) with intranasal oxygen insufflation are the most frequently administered forms of therapy. In those foals with severe hypercapnia in addition to hypoxemia, mechanical ventilation may be necessary.
Gastrointestinal Involvement
Diarrhea or enteritis also is common in septic foals, with a reported incidence of 16% to 38%.12,26,62,63 In a study from Ohio State University, foals with Actinobacillus spp.–induced bacteremia were six times more likely to have diarrhea than those with other isolates.12 With or without enteritis, septic foals also may display signs of ileus or colic. Most of these problems resolve with symptomatic treatment and systemic improvement. The clinician must carefully monitor fluid, electrolyte, and acid-base status in foals with diarrhea and replace ongoing losses. Options for analgesic therapy in colicky foals are somewhat limited; flunixin meglumine should be used cautiously because of the potential for gastric ulceration. Opiates such as butorphanol provide a reasonable short-term option for analgesia in such foals.
Umbilical Involvement
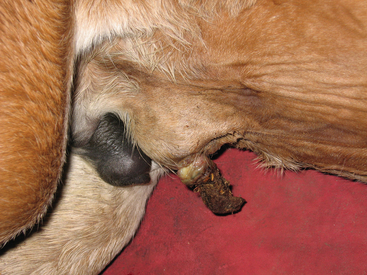
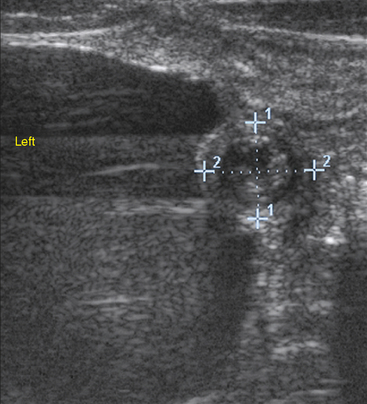
Omphalitis refers to infection of umbilical structures (Fig. 6-3). Umbilical remnant infections are considered to be a common source of continued bacterial shedding and have been reported to occur in 13% of septic foals.63 Ultrasonographic evaluation of these structures is critical because external signs (pain, heat, and swelling) are frequently absent (Fig. 6-4). Treatment options include long-term antibiotic therapy or surgical resection. Many septic foals will develop a patent urachus without involvement of other structures; the reported incidence in septic foals is 21%.63 This problem will often resolve with continued antibiotic therapy, with or without topical therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree