N
Nasal Flush
OVERVIEW AND GOALS
• To obtain cells and/or tissue for diagnostic sampling of intranasal inflammation, infection, or neoplasia
CONTRAINDICATIONS
• High-risk anesthetic animals (i.e., severely compromised metabolic, cardiac, or neurologic disease)
EQUIPMENT, ANESTHESIA
• A 60-mL feeding tip syringe; the larger the diameter of the syringe tip, the more forceful the water jet can be generated by pushing on the plunger.
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO AVOID
• Improper placement or insufflation of the cuffed endotracheal tube, thus allowing water to enter the trachea of the anesthetized animal.
• Unevenly distributed or insufficient gauze, allowing the diagnosis (cells and tissue) to be flushed back into the esophagus.
• Using loose 4 × 4 gauze sponges—some may be forgotten after the procedure is finished and occlude the larynx or trachea.
PROCEDURE
• Place damp gauze dorsal to and on both sides of the endotracheal tube as far caudally as possible, past the caudal edge of the soft palate.
• Gently insert lubricated end of syringe into the ventral meatus of the nose as far as it will go. This is most easily achieved by pushing the philtrum of the nose dorsally while aiming the tip of the syringe toward the septum. Slide into the nasal passage without using force.
• While holding the syringe firmly in place, briskly empty the 60 mL of saline into the nasal passage using moderate pressure on the plunger.
• Repeat several times for each nostril; other towel may be used to control spillage if saline flows from mouth.
POSTPROCEDURE
• Once the gauze is removed, examine the animal’s mouth and pharynx for any material that was flushed out of the nose but not trapped into the gauze.
Nasal Infusion of Clotrimazole
INDICATIONS
Dogs with confirmed nasal aspergillosis; confirmation requires either histopathologic confirmation of fungal hyphae in nasal tissues or at least two of the following: positive Aspergillus fumigatus serum titer, positive Aspergillus culture, radiographic/CT scan findings suggestive of fungal rhinitis (turbinate loss, fungal granulomas; see p. 96).
CONTRAINDICATIONS
• Cribriform plate erosion/damage secondary to fungal rhinitis. If advanced imaging (CT scan or MRI) of the cribriform plate is not available, owners should be warned that cribriform integrity has not been assessed. Topical therapy in animals with known cribriform damage has not been reported.
• Bulky fungal granulomas within the frontal sinus. Massive fungal disease within the frontal sinuses often recurs following topical therapy. It is assumed that the treatment does not adequately penetrate the center of these granulomas. Either rhinoscopic or surgical (sinusotomy) removal of bulky disease, followed by topical clotrimazole, should be considered.
EQUIPMENT, ANESTHESIA
• Two 12 Fr Foley catheters, one 24 Fr Foley catheter, and two 10 Fr polypropylene or red rubber catheters
PROCEDURE
• Pass a 24 Fr Foley catheter orally and then into the nasopharynx with the aid of a long needle holder. Bend the Foley catheter tip 180○ so that the tip flips and comes to be seated dorsal to the soft palate. While palpating through the soft palate, fill the Foley balloon with air. Withdraw the balloon if necessary until it is just caudal to the hard palate. Place laparotomy sponges in the oropharynx to prevent caudal migration of the balloon.
• Pass a 10 Fr polypropylene or red rubber infusion catheter through each nostril and into each dorsal meatus (premeasure to the medial canthus).
• Place a 12 Fr Foley catheter into each nostril to prevent solution from leaking out the nostrils. Place a single nylon suture in each nostril to prevent rostral balloon migration. Fill balloons with air until obstruction occurs.
• Fill two 60-mL syringes with the 1% clotrimazole solution (Lotrimin solution [Schering Corp., Kenilworth, New Jersey]). Administer 60 mL per side each hour via the infusion catheters.
• Position the animal’s head during infusion: 15 minutes dorsal, 15 minutes left lateral, 15 minutes right lateral, and again 15 minutes dorsal. The animal’s body should remain in dorsal recumbency.
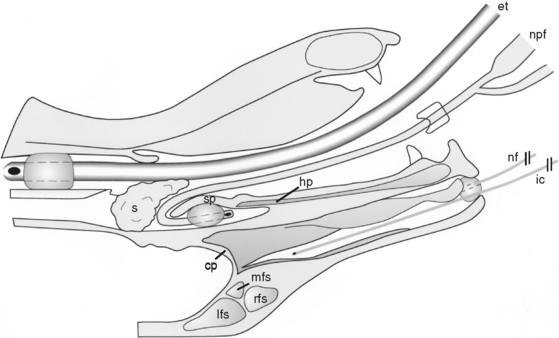 NASAL INFUSION OF CLOTRIMAZOLE Sagittal section of an anesthetized dog in dorsal recumbency. Image shows position of endotracheal tube (et), nasopharyngeal Foley catheter (npf), pharyngeal sponges (s), infusion catheter (ic), and rostral nasal Foley catheter (nf) in relation to the hard palate (hp), soft palate (sp), cribriform plate (cp), rostral frontal sinus (rfs), medial frontal sinus (mfs), and lateral frontal sinus (lfs).
NASAL INFUSION OF CLOTRIMAZOLE Sagittal section of an anesthetized dog in dorsal recumbency. Image shows position of endotracheal tube (et), nasopharyngeal Foley catheter (npf), pharyngeal sponges (s), infusion catheter (ic), and rostral nasal Foley catheter (nf) in relation to the hard palate (hp), soft palate (sp), cribriform plate (cp), rostral frontal sinus (rfs), medial frontal sinus (mfs), and lateral frontal sinus (lfs).(Reprinted with permission from Mathews KG, Koblik PD, Richardson EF, et al: Computed tomographic assessment of noninvasive intranasal infusion in dogs with fungal rhinitis, Vet Surg 25:309–319, 1996.)
ALTERNATIVES AND THEIR RELATIVE MERITS
• Orally administered antifungal drugs may be used in place of intranasal infusion of clotrimazole or as an adjunct to topical therapy. Itraconazole (5 mg/kg, q 12 h PO with food, for at least 2 months) has been associated with the greatest response rate (60%-70%) and requires intermittent monitoring of liver enzymes. A single 1-hour infusion of clotrimazole is equally effective if the medication is injected via catheters placed in dorsal sinusotomies.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree