CHAPTER 59 Myopathic Disorders
Feline skeletal muscle is distinctively different from skeletal muscle in dogs. Skeletal muscle in dogs is composed of slow- and fast-twitch fatigue-resistant myofibers, whereas muscle in cats also includes fast-twitch fatigable myofibers. This fiber type enables cats to be superior sprinters while utilizing energy from glycogen in anaerobic pathways for intense exercise. Slow- and fast-twitch fatigue-resistant fibers utilize mitochondrial fatty acid oxidation primarily, which is ideal for endurance or sustained exercise.1
Diseases affecting normal skeletal muscle function in cats can be categorized as acquired or inherited (Box 59-1). The hallmark clinical sign of any disorder affecting muscle function is weakness. Exercise-induced weakness often is characteristic. The primary gait manifestation for most feline myopathic disorders is a stiff, short-strided, or stilted gait with decreased ability to jump. Somewhat unique to cats affected with myopathic disease, as well as other neuromuscular diseases, is a tendency to develop weakness of the cervical paraspinal extensor muscles, causing cervical spinal ventroflexion or inability to elevate the head and neck (Figure 59-1). Lateral movement of the head and neck seems to be spared. Generalized muscle atrophy may be apparent in some cats affected with myopathies. In contrast, muscle hypertrophy is an abnormality specific to feline dystrophin-deficient muscular dystrophy. Myalgia may be present with myopathy.
In general, distinguishing myopathies clinically from other neuromuscular diseases that involve the motor unit may be difficult based on physical and neurological examinations. Typically, general proprioception and spinal reflexes are not affected in muscle disease. Muscle tone may or may not be decreased. Peripheral neuropathy and radiculopathy often result in hyporeflexia and hypotonia. Diseases involving the neuromuscular junction may or may not affect muscle tone and spinal reflexes. Myasthenia gravis often presents very similarly to myopathy, and affected cats have intact spinal reflexes and muscle tone. Diseases of the motor unit considered as differential diagnoses for generalized weakness in cats are shown in Box 59-2. Further diagnostic testing will assist with establishing a more definitive diagnosis.
Box 59-2 Feline Neuromuscular Diseases
ACQUIRED MYOPATHIES
INFLAMMATORY MYOPATHIES
Infectious
Protozoal
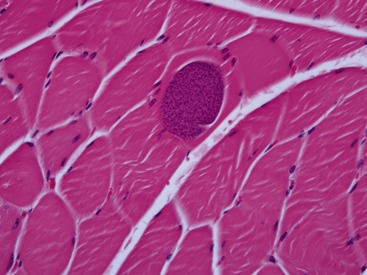
Infections resulting in clinical signs of polymyositis in cats most commonly are parasitic and involve toxoplasmosis. Although kittens can be affected, most affected cats are at least 1 year of age.2 Clinical signs vary among weakness, reluctance to move, and muscle hyperesthesia. Clinical signs of systemic infection often predominate to include anterior uveitis, chorioretinitis, central nervous system dysfunction, respiratory signs, and gastrointestinal disease. Fever and weight loss are common. Hematological and serum chemistry abnormalities typically are present and may include mild nonregenerative anemia, neutrophilia, lymphocytosis, eosinophilia, hyperglobulinemia, and elevations in bilirubin and hepatic enzyme activity. CK activity may be normal or mildly to moderately elevated. Presumptive diagnosis is based on history, clinical signs, serological testing, and response to therapy. In a retrospective study of 15 cats with toxoplasmosis, serum IgM titers were positive in 14 cats, and serum IgG titers were positive in nine of the affected cats.2 In the convalescent phase of the disease, nine cats still had positive IgM titers. Four cats did not develop IgG titers in either the acute or convalescent phases of infection. Definitive diagnosis is based on identification of encysted parasites within muscle biopsy sections; parasites are found in bradyzoite form, and usually are associated with a mild to moderate granulomatous inflammatory reaction (Figure 59-2). Multifocal disease distribution of parasitic migration makes microscopic localization difficult, and the decision to initiate treatment should not be based on absence of organisms in muscle tissue. Clindamycin, 10 mg/kg PO q8h for a minimum of 4 weeks, is the treatment of choice in cats with clinical toxoplasmosis.3 Response to therapy and prognosis is considered to be good if treatment is initiated early in the disease course. Cats with concurrent feline immunodeficiency virus (FIV) or feline leukemia virus (FeLV) infection, however, may be more difficult to manage, with likely recurrences. A compromised immune system is a likely factor in disease pathogenesis in cats with FeLV and FIV infection.
Sarcocystosis has been reported sporadically in immunocompromised cats with cancer and in those patients receiving chemotherapy.4 Although infection with Neospora caninum has been induced experimentally, natural infection involving this organism in cats has not been reported.5
Bacterial
Clostridial myopathy often is associated with muscle injury, penetrating wounds, surgical procedures, and muscular injections. Cats generally are resistant to the effects of tetanus toxin. Development of tetanus secondary to infection with Clostridium tetani, focal or generalized, can be delayed for as long 3 weeks after the inciting wound or injury. Although most infections are focal, the infection can be disseminated.6 The predominant clinical sign is severe pain. Physical examination findings may include crepitus, swelling, and hyperesthesia of affected regions. Radiography may reveal subcutaneous gas. Histopathological findings include extensive muscle necrosis and the presence of gram-positive bacteria. Diagnosis is confirmed with positive results on anaerobic cultures. C. tetani, C. sporogenes, C. septicum, and C. chauvoei have been isolated from affected cats.6,7
If not treated early, infection often is fatal. Treatment involves aggressive surgical débridement and appropriate antibiotic therapy. Depending on the wound location, flushing with hydrogen peroxide can inhibit clostridial growth. Penicillin G is considered the treatment of choice; however, metronidazole may be more effective.8 (The reader is referred to Chapter 8 in the fifth volume of this series for a complete discussion of localized and generalized tetanus.)
Viral
Myopathic abnormalities including perivascular and pericapillary lymphocytic infiltration and myofiber necrosis, phagocytosis, and regeneration have occurred with FIV infection in a research setting.9 Clinical signs of myopathy did not develop in infected cats; however, periodic elevations in CK were reported during the study. Histopathological abnormalities in these cats were comparable to HIV-1–associated polymyositis reported in human beings.
Idiopathic Polymyositis
Idiopathic polymyositis, presumed to be immune-mediated in origin, has been reported sporadically in cats.10–13 Histopathological examination of muscle specimens from affected cats shows a lymphocytic polymyositis. Similar findings in cats with thymoma and myasthenia gravis raise an index of suspicion for an immune-mediated basis.11,12 Some affected cats respond to corticosteroid therapy and spontaneous remission has been reported in others.
The reported age of onset of clinical signs varies ranging from 3 months to 13 years. There is no apparent breed or gender predilection.10 Clinical signs include weakness, exercise intolerance, and anorexia and dysphagia in a smaller number of cases. Neurological examination findings include generalized weakness manifested as a stiff, stilted gait affecting all limbs, cervical spinal ventroflexion, generalized muscle atrophy, and muscle hyperesthesia. Fever is reported in a few cases. The onset of signs typically is subacute. In the majority of cases the duration of clinical signs prior to presentation is less than 1 week, but can range from 1 day to 4 weeks.10 Serum CK activity can be as high as tenfold above the normal range (26 to 141 IU/L). Liver enzyme (aspartate aminotransferase and alanine aminotransferase) activities can be mildly to moderately increased.3,10 Mild decreases in serum potassium have been reported to occur in some affected cats; however, this is not thought to be associated with the primary disease process. Radiographic abnormalities may include changes consistent with the presence of a thymoma and evidence of decreased esophageal motility.
Histopathological findings on muscle sections include a multifocal distribution of interstitial infiltration of mononuclear cells, consisting predominantly of lymphocytes and macrophages with infrequent neutrophils, and rare eosinophils and plasma cells. Perivascular, endomysial, and perimysial distribution has been observed. Associated myofiber necrosis, regeneration, and internal nuclei also are prominent features.10,12 Of the three patients with thymoma reported to have polymyositis, two of the cats also had myocarditis.
Early reports suggest a fair to guarded prognosis with incomplete response to prednisone and a high likelihood of recurrence of signs. An initial immunosuppressive dose of prednisone (4 mg/kg/day PO) with subsequent taper has been recommended for the treatment of idiopathic polymyositis of cats and dogs.3,14 In the author’s experience, treatment with prednisone at an initial dose of 2 mg/kg PO q12h for 2 to 4 days followed by a tapering course over 4 to 6 months often results in complete remission of clinical signs. The long-term prognosis is good.
Paraneoplastic
Polymyositis also has been described in association with some tumors in cats and dogs. This has been described in greater detail in dogs with malignant tumors including carcinomas and myeloid leukemias.15,16 Thymomas have been associated with paraneoplastic polymyositis in cats. Pathogenesis is thought to be immune-mediated, involving T-cell proliferation against an antigen of the sarcolemma or other muscle component. Thoracic and abdominal radiography and abdominal ultrasound are recommended as part of the diagnostic evaluation when paraneoplastic polymyositis is presumed. This author has observed polymyositis in a cat in association with hepatocellular carcinoma, in addition to polymyositis in association with thymoma.
TOXIN- AND DRUG-INDUCED MYOPATHIES
Several toxins and drugs may induce myopathy. Penicillamine and cimetidine have been associated with polymyositis in the veterinary and human literature.3 Medications such as azathioprine and the somatostatins can result in metabolic myopathic disturbances. Snake and insect toxins, and monensin (ionophore antibiotic) have direct effects on the muscle membrane by affecting transport of sodium and potassium.17 Diuretics, insulin overdose, theophylline, amphotericin B, and glycyrrhetinate can cause hypokalemic myopathy (see the hypokalemic myopathy section of this chapter).
METABOLIC MYOPATHIES
Hypokalemic Myopathy
Severe muscle weakness secondary to hypokalemia is a well-recognized disease in cats. Muscle weakness is one of the earliest signs of potassium depletion, which is attributable to a disproportionately higher loss from muscle compared with other tissues. Hypokalemia can occur from systemic loss, reduced intake, or from a shift of potassium from the extracellular to intracellular space. Although hypokalemic myopathy can affect cats at any age, older cats with some degree of chronic kidney disease are at higher risk. In a review of 186 cats with hypokalemia, cats with severe hypokalemia (< 3.0 mEq/L) were 3.5 times more likely to have chronic kidney disease.18 Other causes of hypokalemia include chronic vomiting, chronic diarrhea, potassium-deficient diets, hyperaldosteronism, metabolic acidosis, metabolic alkalosis, hyperthyroidism, and renal tubular acidosis. Drug-induced hypokalemia has been associated with administration of diuretics, insulin overdose, theophylline, amphotericin B, and glycyrrhetinate.17–19
Muscle weakness associated with hypokalemia results initially from increase in the membrane potential of the myocyte, rendering the cells refractory to depolarization. The membrane eventually becomes permeable to sodium ions, and membrane hypopolarization occurs resulting in rapid onset and severe muscle weakness.20 In addition, muscle glycogen metabolism and blood flow during exercise become impaired secondary to hypokalemia.21
Serum potassium levels usually are severely low, reported to be less than 3.1 mEq/L.21 The actual potassium depletion within muscle is more severe than what is reflected in the serum level. In a normal physiological state, serum potassium concentration represents whole body potassium stores. However, as potassium is lost from the body or intake is reduced, there is a disproportionately higher loss from muscle compared with other tissues. Moreover, there also is a disproportionately higher loss from extracellular fluid when compared with tissue. In human beings, serum potassium levels less than 2.0 mEq/L have been correlated with muscle necrosis and rhabdomyolysis.22 Serum CK is mildly to moderately elevated in these cats. In six cats reported as having hypokalemic myopathy, serum potassium values ranged from 2.0 to 3.1 mEq/L (normal, 4.1 to 5.3 mEq/L), and the average serum CK value was 6418 IU/L (normal, 0 to 156 IU/L).21 In a review of 186 hypokalemic cats, 20 patients had serum potassium values below 3.1 mEq/L (normal, 4.1 to 5.3 mEq/L); the average serum CK value of those cats was 2337 IU/L with the highest reported value of 6410 IU/L (normal, 0 to 156 IU/L).18 When potassium levels were restored, serum CK levels returned to within the normal reference range.
Electromyographic findings in affected cats showed increased insertional activity, and abnormal spontaneous activity to include positive sharp waves and fibrillation potentials and bizarre high frequency discharges.21 Muscle biopsies usually were normal; a few cases showed evidence of myofiber necrosis with secondary inflammatory changes.
The most important treatment strategy for hypokalemic myopathy is to normalize the serum potassium level. Oral administration is the safest route. Potassium gluconate at a dose of 2.5 to 5 mEq PO q12h should be administered initially with daily measurements of serum potassium levels.21 Dilution of the potassium gluconate 50 per cent with water is helpful to avoid vomiting. Once the serum potassium normalizes, continued dietary supplementation with 2 to 4 mEq PO q12h in cats with significant renal potassium losses is necessary to prevent recurrence.21 Chronic oral potassium chloride supplementation may cause metabolic acidosis and contribute to further potassium depletion. Long-term management should entail regular monitoring of serum potassium and urinary losses. Cats with severe hypokalemia who are too moribund to receive oral medication should be administered potassium chloride intravenously at a rate of 0.5 to 1 mEq/kg/hr21 until a serum concentration of 3.5 mEq/L is reached. Potassium chloride for IV infusion should be diluted in a balanced electrolyte solution such as lactated Ringer’s. Careful infusion is imperative to avoid phlebitis and fatal heart arrhythmias or asystole.21 Continuous electrocardiographic monitoring is recommended in addition to frequent serum potassium level evaluations. Improvement in strength following treatment is rapid and observed typically within 2 to 5 days. Recovery generally is complete with appropriate therapy. Prognosis is considered good, depending on the underlying cause and/or severity of renal failure.
A unique condition has been reported in young Burmese cats 2 to 12 months of age involving hypokalemic-associated episodic weakness.23–25 This disease is thought to be an inherited channelopathy, and is described in the section on inherited myopathies of this chapter.
Hyperthyroidism
Hyperthyroidism has been associated with muscle weakness. Generalized weakness as a historical complaint is estimated to occur in 12 to 17 per cent of cases, characterized as decreased ability to jump and fatigue associated with exercise.26,27 Cervical spinal ventroflexion has occurred in 1 to 3 per cent of these cases. Extreme muscle weakness usually is associated with concurrent hypokalemia. The prognosis for the weakness is good with treatment of the underlying hyperthyroidism.
Hypernatremia
There is one case report of signs of polymyopathy associated with hypernatremia in a 7-month-old kitten.28 Clinical signs were of 5 days duration and included reluctance to move and cervical ventroflexion. The serum sodium level at presentation was 215 mEq/L (normal, 148 to 165 mEq/L). Signs resolved with correction of the electrolyte imbalance and recurred in association with relapse of the hypernatremia. Long-term management with a low sodium diet was effective in controlling clinical signs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree