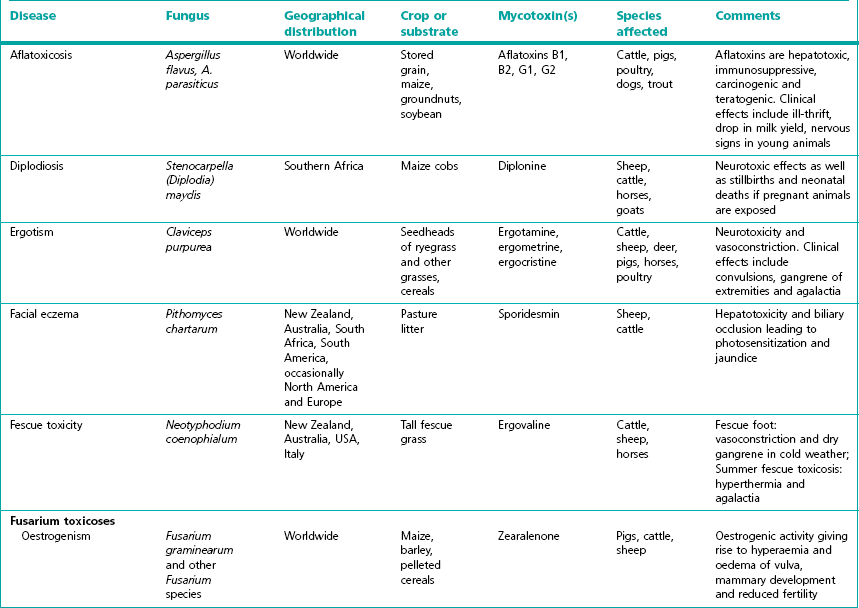
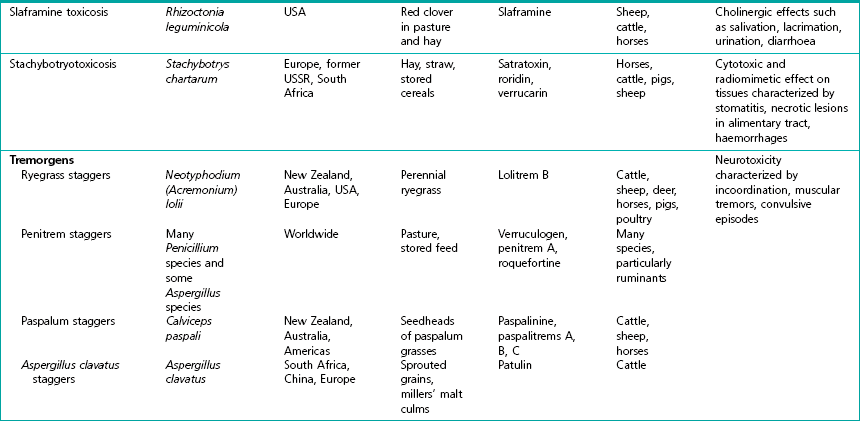
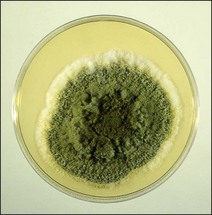
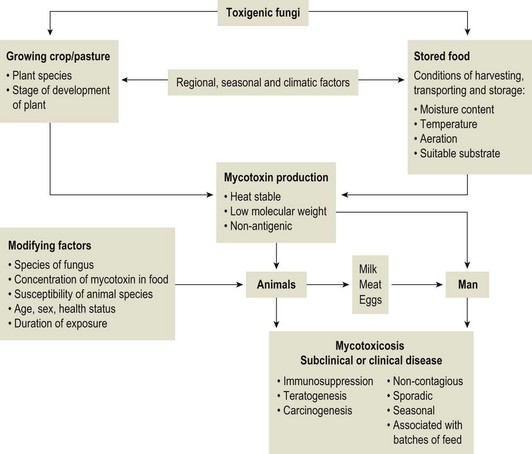
Chapter 44 Mycotoxicoses are diseases in which many factors interact. Animals vary widely in their susceptibility to mycotoxins. Younger animals tend to be more susceptible than adults. There is also considerable variation in species and individual susceptibility. The conditions which favour mycotoxin production and the factors which influence the severity of mycotoxicoses are summarized in Figure 44.1. Since mycotoxins are most likely to be concentrated in highest amounts in stored feeds, groups of animals at risk include poultry, pigs, dairy and feedlot cattle fed on contaminated feed. In some countries, particularly New Zealand, mycotoxicoses such as ‘facial eczema’ are associated with standing pasture and accordingly prevailing climatic conditions determine the occurrence of disease. Figure 44.1 Factors influencing the production of mycotoxins on growing crops or stored foods and the occurrence of myctoxicoses in animals and man. As mycotoxins are low-molecular-weight, non-antigenic substances in their naturally occurring forms, acquired immunity does not occur following exposure. Many of the major mycotoxins are heat-stable and consequently can retain their toxicity after processing temperatures used for pelleting or other milling procedures. Each fungal toxin if present in the diet at a sufficient concentration usually affects specific target organs or tissues. Secondary mycotoxic disease may be more difficult to recognize because low levels of toxin intake may not result in a specific mycotoxicosis but in a heightened susceptibility to intercurrent infections due to immunosuppression. Some of the more important characteristics of mycotoxins are presented in Box 44.1. The severity of mycotoxicoses in animals and their clinical recognition is determined by many factors including the species of toxigenic fungus, the concentration of mycotoxin in the food, the age, sex and health status of the exposed animal, the target organ or tissue affected and the duration of exposure to contaminated feed. The features which characterize mycotoxic diseases include sporadic and seasonal occurrence, lack of transmissibility, association with certain batches of stored food or particular types of pasture, and disappointing response to drug treatment effective against infectious diseases (Box 44.2). Clinical and laboratory procedures may demonstrate pathological changes in the animal, characteristic of a particular intoxication. Clinical diagnosis can be complicated by the presence of a number of toxigenic fungal species on a food source. Diagnosis of a mycotoxicosis requires the demonstration of biologically effective concentrations of the fungal toxin in the feed available to the animal, or in the animal’s tissues, secretions or excretions. Some advances have been made in the application of molecular techniques for the detection of toxigenic fungi (Seifert & Levesque 2004, Mule et al. 2005). Table 44.1 summarizes the principal features of those mycotoxicoses which can be recognized clinically. The level of subclinical and chronic disease associated with the consumption of fungal toxins in feed is difficult to quantify but is certainly of economic and public health significance. Aflatoxins are a group of approximately 20 related toxic compounds produced by some strains of Aspergillus flavus (Fig. 44.2), Aspergillus parasiticus and a number of other Aspergillus species during growth on natural substrates including growing crops and stored food. These fungi are ubiquitous, saprophytic moulds which grow on a variety of cereal grains and foodstuffs such as maize, cottonseed and groundnuts. About half of the strains of A. flavus and A. parasiticus are toxigenic under optimal environmental conditions. High humidity and high temperatures during preharvesting, harvesting, transportation and storage, as well as damage to field crops by insects, drought and mechanical injury during harvesting favour the growth of A. flavus and toxin production. Although other fungi such as Penicillium species and Rhizopus species, are capable of producing aflatoxins, their relevance to livestock production has not yet been established. The name ‘aflatoxin’ derives from Aspergillus(a-), flavus (fla-) and toxin. A number of rapid, economical analytical methods are available for determining aflatoxin levels in a wide range of agricultural food products. Thin-layer chromatography (TLC) is relatively inexpensive and a number of samples can be analyzed simultaneously. The chromatogram is viewed under ultraviolet light for blue or green fluorescent spots that agree in colour and location with internal and external standards (Fig. 44.3). Many different TLC methods have been reported including two-dimensional chromatography for differentiating co-extracted substances. Mini-column detection methods are employed for rapid screening procedures. Although more expensive, high-performance liquid chromatography (HPLC) is easier, faster and gives more sensitive and more reproducible results than TLC. Figure 44.3 Thin-layer chromatography plate of aflatoxins under ultraviolet light showing blue, B2 (extreme left) and green, G2 (extreme right) fluorescence.
Mycotoxins and mycotoxicoses
Characteristics of Mycotoxins
Mycotoxicoses
Aflatoxicosis
Laboratory investigation of outbreaks

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mycotoxins and mycotoxicoses
Only gold members can continue reading. Log In or Register to continue