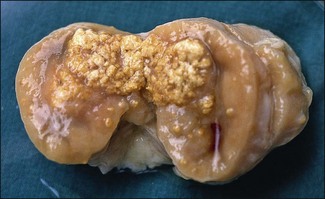
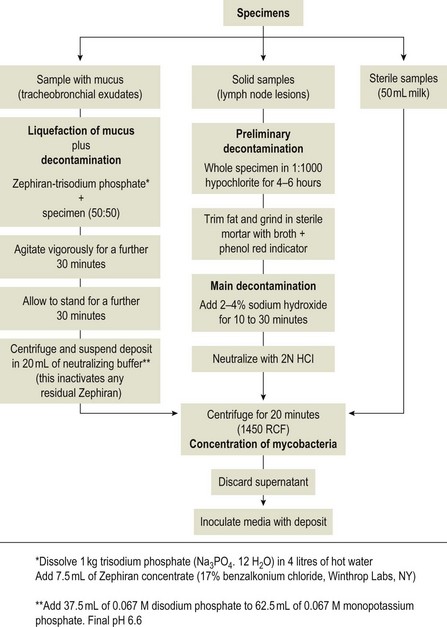
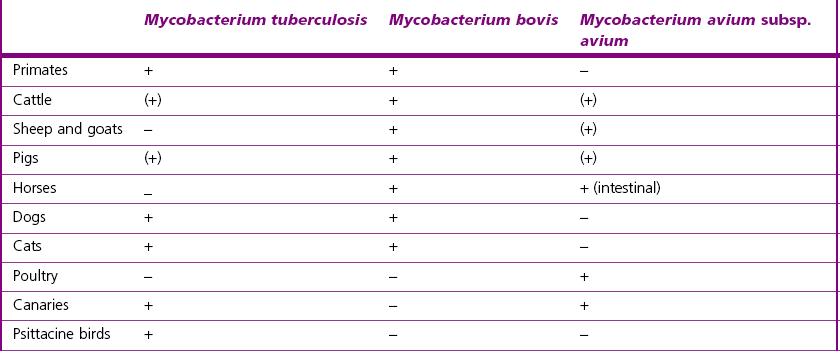
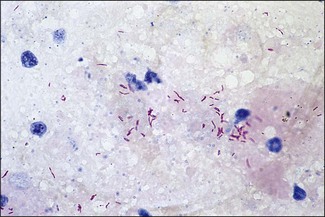
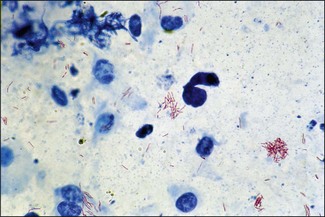
Chapter 11 Runyon (1959) grouped the atypical mycobacteria on the basis of pigmentation, colonial morphology and growth rate. The scotochromogens are those that produce yellowish-orange pigments whether incubated in the light or in the dark. The photochromogens will produce pigment only if exposed to light. For practical purposes, the slow-growing mycobacteria are defined as those that require over seven days’ incubation, under optimal conditons, to produce easily seen colonies and the rapid growers as those requiring less than seven days. Although not all recently isolated species fit within the Runyon scheme, it is still a useful method of categorizing the atypical mycobacteria. The source of the pathogenic mycobacteria is usually infected animals. Mycobacterium bovis is excreted in respiratory discharges, faeces, milk, urine and semen. M. avium subsp. avium and M. avium subsp. paratuberculosis are shed in faeces while M. tuberculosis is mainly shed in respiratory discharges. Tuberculosis is typically a disease of captivity or domestication. However, wild animal reservoirs of M. bovis occur, such as badgers in Europe, brush-tailed opossums in New Zealand, Cape buffalo in East Africa, wild boar in Spain and deer in Europe and America. These hosts may be important in maintaining infection in cattle populations and interfere with the success of eradication programmes. So-called spillover hosts which can be infected by M. bovis but which do not maintain infection in the absence of contact with cattle may also be important sources of infection in some situations (Corner 2006). Table 11.1 gives the mycobacterial diseases that occur in animals and Table 11.2 indicates the susceptibility of domestic animals and birds to the mycobacteria that cause tuberculosis. Table 11.2 Susceptibility of animals to the mycobacteria that cause tuberculosis + = susceptible, (+) = slightly susceptible or may become sensitized, − = resistant to infection The pathogenesis of M. bovis infection in cattle involves the interplay of both host and bacterial factors. Genetic susceptibility of the host is thought to be important in determining the outcome of infection (Allen et al. 2010) as is magnitude of the infectious dose and route of infection. Infection is usually via the respiratory and intestinal tracts. However, the number of organisms required to produce infection in cattle is many times greater via the oral compared to the respiratory route. The organism has not been shown to produce toxic factors, rather virulence appears to reside largely in the lipids and waxes of the cell wall. Mycosides, phospholipids and sulpholipids are thought to protect the tubercle bacilli against phagocytosis. Glycolipids cause a granulomatous response and enhance the survival of phagocytosed mycobacteria. Waxes and various tuberculoproteins induce the delayed-type hypersensitivity detected in the tuberculin test. In previously unexposed animals, multiplication of the mycobacteria occurs within macrophages as phago-lysosome fusion is inhibited. Resistance to phagocytic killing allows continued intracellular and extracellular replication. Infected host cells and mycobacteria can reach local lymph nodes and from there may pass to the thoracic duct followed by general dissemination. After the first week, cell-mediated immune reactions begin to modify the host response and activated macrophages are able to kill some mycobacteria. The cytokines TNF-α and IFN-γ are essential in the activation of macrophages and are produced by both the innate and adaptive immune responses. The aggregation of macrophages contributes to the formation of a tubercle or granuloma (Fig. 11.1) and a fibrous layer may encompass the lesion. Caseous necrosis occurs at the centre of the lesion and this may proceed to calcification (cattle) or liquefaction. Once cell-mediated immunity is established, lymphatic spread is retarded but T-lymphocyte-mediated reactions cause tissue damage and mycobacterial spread occurs by contiguous extension or via the erosion of bronchi, blood vessels or viscera to new areas. Haematogenous dissemination may produce miliary tuberculosis in animals such as deer, characterized by multifocal granuloma formation in major organs or on serosal surfaces. Cell-mediated immune phenomena are involved in the pathogenesis of disease caused by M. avium subsp. paratuberculosis. The organisms are found within macrophages, which are unable to kill them, in the submucosa of the ileocaecal area and adjacent lymph nodes. The ileum and colon are usually involved with extension to the rectum. The mucous membrane becomes thickened and permanently corrugated as a result of cellular infiltration. Lesions may be of either of two types, multibacillary (lepromatous) or paucibacillary (tuberculoid) and appear to be correlated with host immune response. Extensive pathology is correlated with high levels of IL-10 production whereas subclinical disease is associated with increased IFN-γ production (Sweeney et al. 1998, Khalifeh and Stabel 2004). Clinical signs include profuse diarrhoea and the disease is progressive, leading to emaciation and death. Diarrhoea may not necessarily be seen in sheep and goats. Mortality is ultimately caused by malabsorption of nutrients and loss of protein into the intestine. The Ziehl–Neelsen (ZN) stain is used to stain smears from lesions and other specimens. The mycobacteria appear as slender, often beaded, red-staining rods against a blue background (if methylene blue is the counter-stain). These can only be visualized if at least 5 × 104 mycobacteria/mL of material are present. The numbers of M. bovis are often low in bovine specimens but lesions of avian tuberculosis in poultry (Fig. 11.2) and M. bovis lesions in animals such as deer (Fig. 11.3) and badgers usually yield large numbers of mycobacteria. Figure 11.2 Mycobacterium avium in a ZN-stained smear of material from a tubercle in a pigeon demonstrating the numerous, slender, red-staining rods characteristic of avian tuberculosis. (×1000) Figure 11.3 Mycobacterium bovis in a ZN-stained smear of material from a tubercle in a deer. The slender, beaded, red-staining (ZN-positive) rods tend to be more numerous in lesions from deer and badgers compared to the low numbers in bovine lesions. (×1000) Details of isolation procedures for M. bovis are given in Cousins (2009). Several preliminary procedures are necessary in order to recover the comparatively slow-growing mycobacteria: • Selective decontamination to significantly reduce the number of fast-growing contaminant bacteria. • Digestion or liquefaction of mucus. Mucin-trapped mycobacteria in specimens, such as tracheobronchial exudates, may not be available for growth in cultures. • If mycobacteria are present in small numbers they must be concentrated to allow detection by stained smears and culture. This concentration can be done by centrifugation. Mycobacteria are comparatively resistant to acids, alkalis and quaternary ammonium compounds. Decontaminating agents, such as 5% oxalic acid, 2–4% NaOH and 1% quaternary ammonium compound can be used on specimens. The mildest decontaminatng procedure that gives sufficient control over contaminants is desirable but even under optimal conditions 80–90% of the mycobacteria in the specimen may be killed by the decontaminating agent. Each laboratory will need to find the best compromise between destruction of contaminants and survival of sufficient mycobacteria for isolation. Examples of the procedures for recovering mycobacteria from specimens are given in Figure 11.4. The egg-based Lowenstein–Jensen and Stonebrinks media are most commonly used in veterinary bacteriology. Lowenstein–Jensen medium can be obtained commercially and the formula and method of preparation of Stonebrinks medium is given in Appendix 2. The media are prepared as solid slants in screw-capped bottles. Malachite green dye (0.025 g/100 mL) is commonly used as the selective agent. Mycobacterium tuberculosis, M. avium and many of the atypical mycobacteria require glycerol for growth. However, glycerol is inhibitory to M. bovis while sodium pyruvate (0.4%) enhances its growth. Thus media with glycerol and without glycerol (but with Na pyruvate) should be inoculated. The media can be made more selective by the addition of cycloheximide (400 µg/mL), lincomycin (2 µg/mL) and nalidixic acid (35 µg/mL). Each new batch of culture medium should be inoculated with stock strains of mycobacteria to ensure that the medium supports satisfactory growth. The inoculated media may have to be incubated at 37°C for up to eight weeks for the mycobacteria in the tuberculosis group. Mycobacterium tuberculosis and M. avium prefer the caps on the culture media to be loose while M. bovis grows best in airtight containers. Liquid culture media may also be used for the isolation of mycobacteria and commercially available systems suitable for use in specialized laboratories processing large numbers of samples have been developed. Detection of growth of mycobacteria in these systems is based on radiometric or fluorometric methods. Examples include the BACTEC™ and MGIT™(Becton Dickinson) systems.
Mycobacterium species
Runyon’s Groups
Natural Habitat
Pathogenesis
Mycobacterium bovis
Mycobacterium avium subspecies paratuberculosis
Laboratory Diagnosis of Mycobacteria Causing Tuberculosis and Atypical Mycobacteria
Direct microscopy
Isolation
Media for the mycobacteria
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mycobacterium species
Only gold members can continue reading. Log In or Register to continue