Chapter 29 Mycobacteriosis in Amphibians
Mycobacteriosis has a worldwide distribution in humans and animals. The genus Mycobacterium comprises 130 species and 11 subspecies. recognized by the list of prokaryotic names with standing in nomenclature (LPSN). These all share the characteristic morphologic features of gram-positive, aerobic, acid- and alcohol-fast bacteria—nonmotile 1- to 10-µm long rods.7 The pathogenicity of different mycobacteria varies significantly and, for practical purposes, we may differentiate two main groups, the Mycobacterium tuberculosis complex and atypical mycobacteria, including the Mycobacterium avium complex. The most familiar species is M. tuberculosis, the causative agent of human tuberculosis, responsible for the death of over 2 million people each year. Atypical mycobacteria have a very important place in human and animal health as well, especially in immunodepressed hosts.
Mycobacteriosis is the oldest known infectious disease of amphibians, with a history dating back to the latter part of the 19th century. Robert Koch isolated M. tuberculosis in 1882. In 1905, Küster reported the first case of amphibian mycobacteriosis in three frogs (Rana temporaria).12 To our knowledge, only atypical mycobacteria, or MOTT (mycobacteria other than M. tuberculosis complex), have been isolated in amphibians.
Epidemiology
Mycobacteria are ubiquitous in aquatic environments or as soil saprophytes and are found commonly in nature; they are likely to be seen in most, if not all, amphibian facilities. In that way, the epidemiology of mycobacterial infection in amphibians is different from that seen in birds and mammals—the clinician must always include mycobacteriosis in the differential diagnosis. Mycobacterial disease has been reported among exhibited amphibians and in laboratory models. A review in 2001 described 31 species of amphibians, representing nine families, affected by mycobacteriosis. Since then, this list has likely expanded considerably.19 An increasingly diverse array of Mycobacterium spp. has been isolated from frogs in recent years, supplanting the paradigm of M. marinum, M. fortuitum, and M. xenopi as the only causative agents adequately described. A number of them have been isolated, including M. abscessus, M. chelonae, M. fortuitum, M. marinum, M. gordonae, M. ranae, M. thamnospheos, Mycobacterium avium, M. xenopi, and M. szulgaï.2 A newly described Mycobacterium species, closely related to M. ulcerans and M. marinum, has been isolated from Xenopus tropicalis.14 M. liflandii is characterized by the presence of insertion sequences IS2404 and IS2606, as well as by the production of the toxin mycolactone. Both mycolactone production and the presence of IS2404 were thought to be restricted to M. ulcerans.18,20 The last part of this chapter will discuss M. liflandii in more detail.
Natural transmission of mycobacteria in amphibians is poorly understood. Mycobacteria are ubiquitous in the environment. Water and associated biofilms are natural habitats for many of them, so water-borne transmission seems likely in most amphibians. Vectors must also be considered—borne mycobacteria are known to infect a number of aquatic organisms and survive and replicate in various protozoan hosts.9 Vertical transmission of mycobacteria has been suggested in fish, but not yet in amphibians.5 As a rule, it is believed that they infect animals through defects in the integument or by ingestion (contaminated food and water).
Although mycobacteriosis is considered to be precipitated by stress and occurs in immunodepressed host, specific factors leading to disease outbreaks are seldom defined and appear to vary among systems. The initial dose influences disease outcome, as evidenced by a study in the leopard frog (Rana pipiens), in which M. marinum produces chronic disease with a low initial innoculum and acute or subacute disease with higher infecting doses. The minimum number of persistent organisms required to stimulate granuloma formation appears to be approximately 10.4 Furthermore, the study showed that M. marinum causes a chronic, granulomatous, nonlethal disease in immunocompetent frogs. However, in experimental frogs immunosuppressed with hydrocortisone, infection results in an acute, fulminant, lethal disease.17 There have been no reports describing mycobacteriosis associated with large-scale mortality in wild amphibians, although I have studied several large epizootics attributed to the disease in amphibian research facilities.
Pathogenesis
Mycobacteria induce granulomatous reactions in human and animal hosts. With the exception of M. ulcerans and others, mycolactone-producing mycobacteria (MPM), pathogenic mycobacteria in vertebrates are predominantly intracellular parasites of phagocytes. Several studies in frogs and fishes have suggested that multiple intracellular survival strategies may be used.8 MPM are unique among mycobacterial pathogens in that they are mainly extracellular and secrete mycolactone. Mycolactone causes apoptosis and necrosis of cultured cells in vitro and inhibits proinflammatory cytokines and phagocytosis. M. ulcerans is responsible for the progressive necrotizing skin disease seen in humans afflicted with Buruli ulcer.10,21 A number of variant mycolactones (A to D) have been identified in M. ulcerans from different geographic locations. Mycolactone E is the mycolactone that has been recovered from amphibians with M. liflandii infection.14
Clinical Signs and Pathology
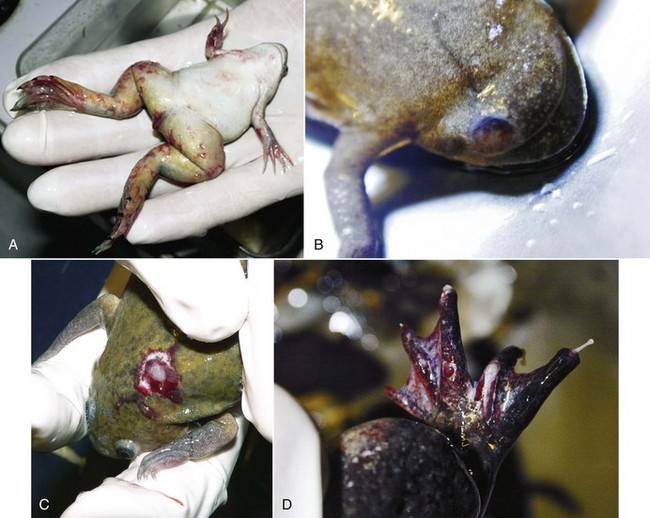
Mycobacteriosis is predominantly a chronic granulomatous infection. Although insidious chronicity with no clinical signs is the rule, peracute and acute cases may occur, reflecting the heterogeneous biologic behaviour of the various mycobacterial species and the heterogeneity of host responses. Acute disease is generally observed in association with high bacterial loads. All amphibian tissues may be involved, including skin, visceral organs, musculature, and skeleton (Fig. 29-1). External clinical signs are not pathognomonic and range from some weight loss to cachexia, granulomatous dermatitis, dermal ulceration, pigmentary changes, abnormal behavior, ataxia, and ascites. At necropsy, granulomas are usually observed throughout the whole body, with or without enlargement of visceral organs such as the spleen, kidney, and liver. With the heterogeneity of host responses, significant variation in the size and structural organization of granulomas may be seen, from highly organized lesions with thick epithelioid layers to poorly organized inflammation. Lesions consist of large chronic inflammation areas, composed of concentric layers of epithelioid cells with a discrete spherical form. Diffuse infiltration of activated macrophages and few lymphocytes with central necrosis may be observed.2 The disease may present as a solitary massive nodule or, more often, as a systemic disease, with granulomas produced in multiple organs or tissues (Fig. 29-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree