Chapter 61 Mushrooms
COLLECTION AND IDENTIFICATION
Mushrooms grow on several different kinds of materials, called substrates. A mushroom growing on wood is lignicolous, one on the ground is terrestrial, one on another plant or animal is parasitic, and one growing on dung is coprophilous. Knowledge of the substrate helps identify the mushroom (Table 61-1). For example, if a mushroom growing out of the side of a stump was identified as an Amanita, a terrestrial mushroom, the mushroom has been misidentified. Try to identify the substrate when possible. Look carefully, an apparent terrestrial mushroom may be growing on underground wood or buried dung!
Table 61-1 Toxic Mushrooms by Growth Substrate
| Growth Substrate | Mushroom |
|---|---|
| Earth | Agaricus xanthodermus |
| Amanita bisporigera | |
| Amanita phalloides | |
| Amanita verna | |
| Amanita virosa | |
| Chlorophyllum molybdites | |
| Clitocybe dealbata | |
| Clitocybe rivulosa | |
| Coprinus species | |
| Cortinarius splendens | |
| Lepiota helveola | |
| Lepiota naucina | |
| Panaeolus campanulatus | |
| Pholiotina filaris | |
| Sarcosphaera crassa | |
| Scleroderma species | |
| Stropheria coronilla | |
| Dung | Panaeolus sphinctrinus |
| Pslocybe cubensis | |
| Wood | Galerina autumnalis |
| Galerina marginata | |
| Gyromitra ambigua | |
| Gyromitra esculenta | |
| Gyromitra infula | |
| Hypholoma fasciculare | |
| Megacollybia platyphylla | |
| Omphalotus olearius |
Data from references 6, 133, 134.
The “seeds” or spores of mushrooms are also an identification aid. The spores are microscopic and may only be seen as a mass. Before dispersal they may be located under the mushroom top or “cap” in structures that look like a pincushion, sponge, or on thin fins of tissue called gills. Some mushrooms do not have a specific cap and bear their seeds inside the mushroom (Figure 61-1). As the mushroom matures and the spores ripen, the surface of the mushroom opens and the spores are blown out, providing a “puffball.” Some spores are borne on cap wrinkles or folds, as in a false morel mushroom (Figure 61-2), or inside pits, like in the true morel mushroom (Figure 61-3).
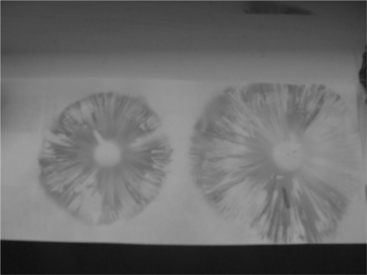
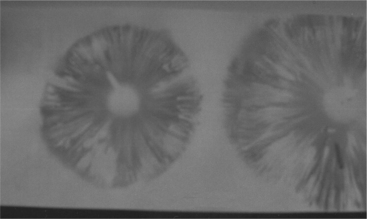
A spore print is also very helpful in identification. A spore print consists of a mass of spores deposited on white paper. The color of the spores is specific to a species and is usually listed in most field guides used for identification. The spore print is obtained by cutting the stem of the mushroom off just under the cap. The mushroom is placed with the gills or spongy area down on a white sheet of paper. The spores fall out of the cap and onto the paper to provide the color. Colors include brown, reddish brown, chocolate brown, white, cream, black, purple-black, green, and yellow. The pattern of the spore print is not important, since it is determined by the shape of a single mushroom and may vary considerably. If the mushroom has greatly raised edges or is irregular in shape, a bowl may be placed over the mushroom to get a better concentration of spores (Figures 61-4 and 61-5).
Many different shapes are described for the various parts of the mushroom. Structures with some importance in poisoning cases are the annulus, universal veil remnants, vulva, and stem and cap shape (Figure 61-6). Imagine a mushroom as it comes out of the ground as an egg. As it increases in size it splits horizontally. In some types of mushrooms the universal veil, or “egg shell,” that once was the outer surface breaks into smaller and smaller pieces, often sticking to the surface of the actual mushroom growing underneath. These spots, or splotches, are called universal veil remnants and are helpful in identifying various species, many of which are poisonous. Part of that outside veil may separate from the cap and stay with the stem to form a ring on the stem, called an annulus. The annulus may be large, small, fleshy, or threadlike, characteristics useful for identification (Figure 61-7). The shape of the stipe (stem) is important; it may be equal in size from top to bottom, wider at the top, or wider at the bottom. The stem base may be directly attached to the mycelium and substrate or enclosed within a cuplike sheath called a vulva. As an example of how these characteristics are used, a poisonous Amanita mushroom looks a lot like an edible Agaricus, but the Amanita has white spores, a vulva, an annulus, and cap “splotches,” (Figure 61-8) whereas the Agaricus has brown spores and an annulus only. The overall color of the mushroom is of some importance, but considerable biological variation may occur which makes identification by color alone hazardous.
If a mushroom ingestion is suspected, thoroughly examine the area where the animals may have been exposed and collect any mushrooms in the area for identification by the veterinarian or a mycologist. Place the mushrooms in paper, not plastic, bags. The most useful collection technique is to place the mushroom with cap gills down on a white sheet of paper that has the site and conditions (e.g., moisture, nearby trees, and substrate) written on it, and then wrap each type of mushroom in wax paper. Certain mushrooms are commonly associated with specific trees. Knowledge of the surrounding trees can aid in identification (Table 61-2). In small animal poisoning cases it may be very difficult to obtain a complete sample set of mushrooms if the animal has ranged over a wide area.
Table 61-2 Mushrooms Found in Association with Various Trees
| Tree Type | Mushroom |
|---|---|
| Alder | Clitocybe candicans |
| Clitocybe clavipes | |
| Inocybe dulcamara | |
| Balsam | Gyromitra esculenta |
| Beech | Amanita citrina |
| Amanita gemmata | |
| Amanita phalloides | |
| Boletus satanas | |
| Clitocybe candicans | |
| Clitocybe clavipes | |
| Cortinarius orellanus | |
| Cortinarius splendens | |
| Entoloma simuatum | |
| Inocybe patouillardii | |
| Ramaria formosa | |
| Birch | Amanita citrina |
| Amanita muscaria | |
| Clitocybe candicans | |
| Clitocybe clavipes | |
| Inocybe dulcamara | |
| Lactarius helvus | |
| Lactarius torminosus | |
| Leccinum atrostipitatum | |
| Leccinum scabrum | |
| Chestnut | Amanita verna |
| Omphalotus olearius | |
| Fir | Amanita pantherina |
| Gyromitra esculenta | |
| Inocybe species | |
| Suillus lakei | |
| Hornbeam | Amanita phalloides |
| Cortinarius orellanus | |
| Entoloma sinuatum | |
| Larch | Clitocybe candicans |
| Fuscoboletinus spectabilis | |
| Sullius cavipes | |
| Madrona | Amanita calyptroderma |
| Amanita muscaria | |
| Oak | Agaricus xanthoderus |
| Amanita citrina | |
| Amanita phalloides | |
| Amanita verna | |
| Boletus satanas | |
| Clitocybe clavipes | |
| Cortinarius orellanus | |
| Entoloma sinuatum | |
| Inocybe dulcamara | |
| Lepiota species | |
| Omphalotus olearius |
| Tree Type | Mushroom |
|---|---|
| Olive | Omphalotus olearius |
| Pines | Amanita citrina |
| Amanita muscaria | |
| Amanita gemmata | |
| Amanita pantherina | |
| Amanita regalis | |
| Armillaria mellea | |
| Armillaria ponderosa | |
| Armillaria zelleri | |
| Chroogomphus vinicolor | |
| Clitocybe candicans | |
| Cortinarius orellanus | |
| Hebeloma crustuliniforme | |
| Inocybe dulcamara | |
| Lactarius helvus | |
| Ramaria pallida | |
| Suillus brevipes | |
| Sullius granulatus | |
| Sullius luteus | |
| Sullius pictus | |
| Poplar (aspen) | Hebeloma crustuliniforme |
| Flammulina velutipes | |
| Lactarius controversus | |
| Leccinum insigne | |
| Pleurotus ostreatus | |
| Spruce | Amanita citrina |
| Amanita regalis | |
| Amanita virosa | |
| Armillaria mellea | |
| Gyromitra esculenta | |
| Lactarius helvus | |
| Ramaria pallida |
Data from references 6, 17, 133, 134.
Small animal poisonings caused by mushrooms are not reported nearly as often as with humans, but the actual exposure is likely underreported. The North American Mycological Association maintains a voluntary reporting system for cases of mushroom poisonings. The most commonly reported animals affected up through the year 2000 were dogs (68 cases) followed by cats (17 cases).1
TOXINS
There are various types of mushroom toxins, most of which have a characteristic set of clinical signs, at least in humans (Table 61-3). The generally held public opinion is that if an animal eats a mushroom, it must be safe, implying that an animal would not eat a toxic mushroom. Such is not the case. Animals and humans can be poisoned by eating fungi. Ingestion of a fungal species by an animal does not make it safe for human consumption. There are actually many cases of toxic mushroom ingestion, especially in cats and dogs, but few of these are reported in the literature (Table 61-4).
Table 61-3 Mushrooms by Toxin Type
| Toxin | Mushroom |
|---|---|
| Amatoxins (cyclopeptides) | |
| Gastrointestinal, renal and hepatic effects | Amanita bisporigera |
| Amanita ocreata | |
| Amanita phalloides | |
| Amanita verna | |
| Amanita virosa | |
| Conocybe filaris | |
| Galerina autumnalis | |
| Galerina marginata | |
| Galerina venenata | |
| Pholiotina filaris | |
| Coprine type — rare | Boletus luridus |
| Clitocybe clavipes | |
| Coprinus atramentarius | |
| Coprinus micaceus | |
| Gastrointestinal irritants | Amanita xanthoderus |
| Amanita volvata | |
| Boletus eastwoodiae | |
| Boletus pulcherrimus | |
| Boletus satanas | |
| Cantharellus floccosus | |
| Chlorophyllum molybdites | |
| Entoloma lividum | |
| Hebeloma crustuliniforme | |
| Gomphus floccosus | |
| Laccaria amethystina | |
| Lampteromyces japonica | |
| Omphalotus illudens | |
| Psalliota campestris | |
| Ramaria Formosa | |
| Rhodophyllus rhodopolius | |
| Rhodophyllus sinuatus | |
| Russula emetica | |
| Scleroderma species | |
| Suillus tomentosus | |
| Tylopilus felleus | |
| Hydrazines | |
| Gastrointestinal, CNS, and RBC effects | Gyromitra ambigua |
| Gyromitra esculenta | |
| Gyromitra gigas | |
| Gyromitra infula | |
| Helvella elastica | |
| Verpa bohemica | |
| Piziza badia | |
| Ibotenic acid/muscimol | |
| Gastrointestinal and CNS effects | Amanita muscaria |
| Amanita pantherina | |
| Amanita strobiliformis | |
| Tricholoma muscarium | |
| Toxin | Mushroom |
|---|---|
| Muscarine —SLUD | Clitocybe dealbata |
| Clitocybe rivulosa | |
| Clitocybe truncicola | |
| Entoloma rhodopolium | |
| Inocybe lacera | |
| Inocybe fastigiata | |
| Chlorophyllum molybdites | |
| Inocybe patouillardii | |
| Inocybe phaeocomis | |
| Inocybe pudica | |
| Mycena pura | |
| Orelline/orellanine — Nephrotoxic | Cortinarius orellanus |
| Cortinarius speciosissimus | |
| Psilocybin/psilocin | |
| Hallucinogenic (CNS) | Conocybe species |
| Gymnopilus species | |
| Panaeolus species | |
| Psilocybe species (many) | |
| Stropharia species | |
Data from references 6, 123, 133.
Table 61-4 Mushrooms Ingested by Dogs and Cats in the North American Mycological Association’s Database 1997–20001,32,60,61
| Mushroom Species | Dog | Cat |
|---|---|---|
| Amanita muscaria | 3 | 1 |
| Amanita pantherina | 7 | 1 |
| Amanitapantherina/muscaria | 4 | |
| Amanita virosa | 1 | |
| Bolbitius vitellinus | 1 | |
| Gyromitra species | 1 | |
| Hypholoma fasciculare | 1 | |
| Scleroderma species | 1 | |
| Trametes versicolor | 1 | |
| Unknown | 15 | 1 |
Cyclopeptides: gastrointestinal, renal, and hepatic effects
Amatoxins inhibit one of two mammalian nuclear RNA poly-merase B forms, which are involved in the transcription of DNA to messenger RNA. They therefore cause inhibition of protein synthesis at the ribosomes.2–5
Amanita and Galerina species are most commonly involved. The Galerina mushrooms are small, dark brown, and grow on wood. Amanita mushrooms come in a variety of colors and have a vulva, an annulus, and universal veil remnants. The primary target organs of the cyclopeptide toxins are the gastrointestinal tract, kidney, and liver. After ingestion of these toxins by a human, there is usually a latent period during which little happens for several hours. The patient then starts to vomit and may have diarrhea and intense abdominal pain for several hours.7–9 The vomiting then subsides, and the patient may seem almost normal. During the next several days, the toxins cause hepatic and renal failure. If the dose has been high enough, the patient dies in the following few days. During this time hepatic enzymes are elevated, and the patient may be jaundiced and anuric from the toxins or from hypovolemia. Tachycardia, acid-base abnormalities, dehydration, hypotension, electrolyte abnormalities, hepatic coma, coagulation abnormalities, hypoglycemia, and shock may occur.10–12 Laboratory tests may show elevations in prothrombin time, Quick’s test values (a measure of plasma prothrombin), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), serum bilirubin, and creatinine.6,13 Amanita smithiana, usually found in the northwestern states, can cause renal tubular necrosis with fewer hepatic signs. Although amatoxins can be detected in the blood using a radioimmunoassay, this test is seldom helpful because the toxins disappear from the blood fairly quickly, 2 to 36 hours in humans.
Several treatments have been tried in humans with moderate success. Decontamination of the gastrointestinal tract is a good idea if the ingestion has occurred within the last few hours. Emesis and/or lavage, along with administration of activated charcoal, may be used. Multiple doses of charcoal, given several hours apart, have been useful in humans because of enterohepatic circulation.14 Good supportive care is crucial, with observation and treatment of renal failure, hepatic coma, acid-base abnormalities, bleeding, hypoglycemia, or dehydration.15 Coagulopathies may require injections of vitamin K1. Blood transfusions may be necessary. Liver transplants have been done in humans but would be rare in animals.
Thioctic acid was once recommended, but it was difficult to obtain and had mixed success. Penicillin G, in human doses of 300,000 to 40 million U/day, has been tried and seems to be of some benefit if given within a few hours of ingestion.7,16,17 Animal experiments seem to indicate that penicillin G reduces hepatic uptake of the toxin. Another agent used is silymarin, derived from the milk thistle Silybum marianum.7,18–25 The flavonolignans in silymarin have been found to be protective in animals. Silymarin is thought to be a free radical scavenger and an inhibitor of the penetration of the amatoxins into liver cells. Human doses are 20 to 50 mg/kg/day given intravenously (not available in the United States) or 1.4 to 4.2 g/day orally.26 When using herbal milk thistle, the concentration of silymarin should be determined from the label and the amount needed for a 1.4 to 4.2 g dose calculated from that. The drugs are usually given for about 4 to 5 days. Often penicillin G and silymarin are used together. Forced diuresis has also been tried with some success.15,27 An antibody against amatoxin has been developed in rabbits but is experimental only.28
ANIMAL EXPOSURES.
A 1-year-old female cat ate an unknown amount of an unidentified Amanita species and depression and weakness, ear twitching, hyperexcitability, poor pupillary response leading to no pupillary response, subnormal temperature (96°F), bradycardia (70 beats per minute), vertical nystagmus, and premature ventricular contractions developed; she eventually died 45 minutes after reaching veterinary care. Necropsy showed severe hepatic damage, massive edema of all tissues, and pieces of mushroom cap and stems in the stomach. The cat had diarrhea, passing large amounts of clear gelatinous material.29,30
Amatoxins are very toxic to dogs, producing fulminant liver failure, renal failure, coagulopathy, and possible death within a few days.31,32 An initial hyperglycemia followed by hypoglycemia developed in beagle dogs that were experimentally given amatoxins. Death occurred in 1 to 2 days. When glucose was administered, an acute hepatic dystrophy developed in the dogs that was fatal in 1 to 2 days. Severe hemorrhages were seen in multiple organs in some subjects, and death caused by renal failure was seen in a few animals.33
Ataxia, listlessness, and petit mal seizures developed in a golden retriever puppy who ingested an unknown amount of Amanita verna. Treatment was attempted with diazepam and lactated Ringer’s solution, but the puppy died 6 hours after the onset of clinical signs.34 A 20-month-old Boston terrier who ingested an unknown amount of Amanita virosa died in approximately 36 hours after experiencing hepatic damage, hypoglycemia, coagulation abnormalities, bradycardia, and shock.35
Fulminant liver failure developed in two dogs with confirmed ingestion of Amanita phalloides and A. ocreata. One died within a day, despite aggressive treatment including hemoperfusion, and the other survived.31
Rabbits appear to be able to nibble on Amanita phalloides without apparent harm. At one time rabbit brains were used as an antitoxin for cyclopeptide poisoning.36
Horses do not commonly eat Amanita mushrooms, but an 18-year-old horse died after apparently ingesting one or more Amanita verna mushrooms. This animal had a brain meningioangiomatosis which may have altered its normal eating behavior.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree