CHAPTER 26 Musculoskeletal Diseases
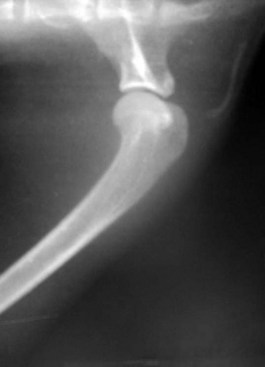
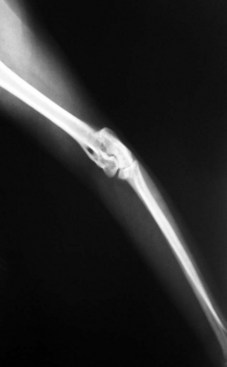
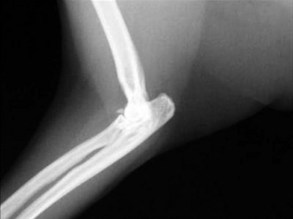
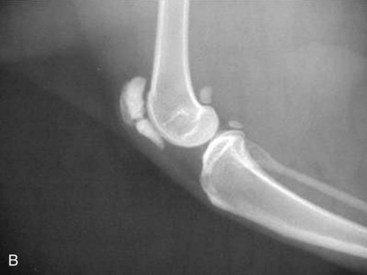
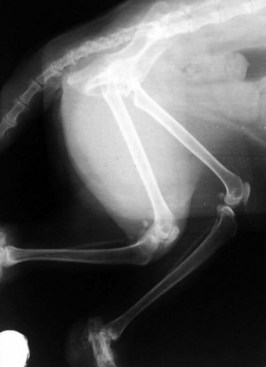
The cat has a number of anatomic and physiologic differences compared with the dog. Some are mere curiosities, whereas others can be extremely significant from a diagnostic perspective.96 The presence of a free-floating clavicle in the cranial shoulder region falls into the category of curiosity, but it is sometimes mistaken for a fracture or foreign body (Figure 26-1). The median nerve and brachial artery pass through the supracondylar foramen on the medial side of the distal humerus in the cat, whereas the same structures lie medial to the humerus in the dog (Figure 26-2). The presence of these vital structures within the humeral metaphysis of the cat restricts the placement of orthopedic hardware in this region. In the condylar region of the distal humerus, there is no supratrochlear foramen in the cat as there is in the dog. This is one of the main reasons that humeral condylar fractures are relatively less common in cats.65 Approximately 40% of cats have a sesamoid bone in the tendon of origin of the supinator muscle on the dorsal surface of the proximal radius (Figure 26-3). This structure may be visible on lateral radiographic projections of the elbow and should not be mistaken for a chip fracture. The round ligament of the femoral head provides significant vascular supply to the femoral head in the cat, which is not the case in the dog. This may be one reason that aseptic necrosis of the femoral head is not described in the cat. The cranial cruciate ligament is larger and thicker than the caudal cruciate ligament in cats, which is the reverse of what is found in the dog. This may be an important factor explaining why rupture of the cruciate ligament is much less common in the cat. The range of motion in the feline shoulder and hip is greater than in the dog, but in the feline carpus and stifle range of motion is less than in the dog. However, supination of the carpus and paw is much greater in the cat and is important in grooming behavior.13,65,96,103
Fractures
Fractures make up a large percentage of musculoskeletal problems in the cat, with the distribution of fractures being somewhat unique to this species. Although both dogs and cats suffer a majority of their fractures in the hind limb or pelvis, this percentage exceeds 70% of all fractures seen in the cat.48 When the 11% to 23% of feline fractures that involve the maxillae, mandible, or facial bones are included, these two regions account for the overwhelming majority of fractures in the cat.
Because most fractures are associated with significant trauma, a thorough evaluation of the entire cat beyond the fracture is essential. Published estimates suggest that as many as 40% of fracture patients also have thoracic trauma, which may affect not only the treatment plan but also the patient’s very survival.13
Mandibular and Maxillary Fractures
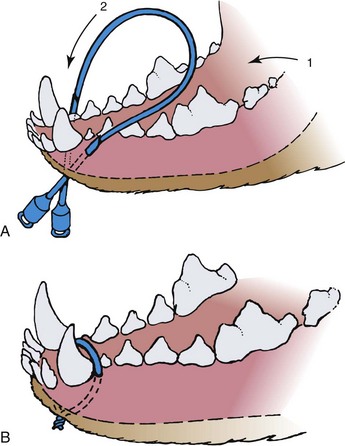
Fractures involving the mandible or maxillae of cats are unique if for no other reason than that they are at least tenfold more common in the cat than in the dog.86 Vehicular trauma and high-rise syndrome are the most common causes of these fractures, which occur when the cat absorbs a face-first impact. Not surprisingly, such trauma is frequently associated with additional injuries, including broken teeth, thoracic injury, head trauma, and forelimb fractures. Fracture of the mandibular symphysis accounts for nearly three quarters of mandibular and maxillary injuries.47,86 Circumferential wiring of the symphysis combined with 3 to 4 weeks of a soft diet followed by wire removal is usually successful (Figure 26-4). At the time of wire removal, some residual mobility may still be present at the symphysis, but this is due in part to the fact that the joint is cartilaginous and is not rigid, even in the normal state. Most patients do well clinically, regardless of the mobility.
Midsagittal splits of the hard palate are another common consequence of frontal facial impact trauma in the cat. Minor splits of 1 to 2 mm usually require no specific repair; however, wider splits should be compressed. This can be accomplished by running surgical wire in a figure-of-eight pattern across the split along the oral surface of the hard palate and around the base of a tooth on each side of the maxillae. Alternatively, a Kirschner wire can be driven across the maxillae between teeth so that the ends of the wire are exposed through the gingiva on either side of the maxillae, just dorsal to the level of the hard palate. A figure-of-eight tension band wire is then placed around each end of the wire and tightened to achieve compression of the palatine split. The Kirschner wire ends can be bent over to prevent trauma to the lips. The hardware is removed after 4 weeks.47
Treatment of more complex mandibular or maxillary fractures can involve many of the same techniques used in dogs, including interdental wiring, intraoral splints, bone plates, and external skeletal fixators. Regardless of the technique, the primary goal is to restore perfect dental occlusion. It bears remembering that the most beautiful surgical repair is of little consequence if the teeth do not fit. An adhesive tape muzzle can be used effectively in the cat despite its short, conical muzzle. A length of tape is wrapped around the muzzle with the adhesive side facing out, caudal to the level of the canine teeth. Care is taken to ensure that the canine teeth interdigitate properly but that there is enough space between the incisor teeth to allow the cat to lap liquid diets and water. Performing the procedure under general anesthesia with an endotracheal tube in place usually provides the proper amount of space. A length of tape is then passed behind the ears and stuck to the adhesive surface of the muzzle wrap on the each side of the muzzle. Another wrap of tape is placed around the muzzle to hold the second length of tape in place. A final length of tape is placed from the head strap on both sides and beneath the cat’s throat to prevent the head strap from pulling up over the ears.47,96 In cases in which fracture comminution precludes surgical reconstruction and stabilization, when financial constraints eliminate a surgical option, or in young kittens whose soft bone and erupting teeth make the use of surgical hardware difficult, a tape muzzle permits the maintenance of normal dental occlusion and often produces surprisingly good results.47,86,96
Patellar Fractures
Traumatic fractures of the patella can occur in cats. If fracture fragments are sufficiently large, they can be stabilized with a pin and tension band wire. Small fragments may be removed. In either case the integrity of the quadriceps muscle and patellar tendon mechanism must be maintained.63 Some cats may be born with bipartite patellas. The radiographic appearance reveals smooth edges to the patellar fragments and frequently a similar appearance bilaterally. The condition is usually an incidental finding but can cause diagnostic confusion in a lame cat.
An additional subset of young adult cats develops fractures of one or both patellae with no history or evidence of trauma. Lameness is acute but usually mild to moderate. Although the etiology is unclear, these have been characterized as stress fractures.62 Evidence for this pathogenesis includes the lack of known trauma in most cases and the presence of radiographic sclerosis of the fracture fragments and often the contralateral patella if it is intact. The fractures are simple transverse, involve the proximal one third of the patella, and are bilateral about half the time. In one survey about half of the contralateral patellae subsequently fractured at a mean time of 3 months.62 Attempts at surgical repair by pin and tension band wire have met with almost universal failure, characterized by iatrogenic fracture of the remaining fragments; hardware failure; or, most often, nonunion. However, most cats regained reasonable function of the limb, with stiffness or intermittent lameness in about half the cases.62 Some of the cats were found to have retained deciduous teeth or delayed dental eruption (Figure 26-5, A). In humans there is a connection between dentinogenesis imperfecta, which involves a number of dental abnormalities, and osteogenesis imperfecta, a condition involving brittle, easily fractured bones that is also seen in cats (discussed later).
Of 34 cats with apparently atraumatic patellar fractures, 10 also had a history of previous, concurrent, or subsequent fracture of other bones (see Figure 26-5, B).62 It may be that some of these cats with patellar fractures have a form of osteogenesis imperfecta. Conservative treatment of these patellar fractures would appear to be the most prudent course, especially if distraction of fracture fragments is mild to moderate. If fragments are significantly distracted, then a circumferential wire may be preferred over an attempt to pass a pin through sclerotic bone. Alternatively, partial patellectomy may be performed. Complete patellectomy does not usually produce satisfactory function.62
Radial and Ulnar Fractures
Fractures of the radius and ulna are relatively uncommon in cats, composing between 5% and 13.8% of feline fractures.84 Further, surgical repair of these fractures, especially when comminuted or open, is associated with a high complication rate.103 This would appear to be primarily due to the cat’s ability to pronate the front limb and paw to a much greater degree than the dog. This increased mobility between the bones means that the standard surgical approach in the dog of stabilizing only the radius, when both bones are fractured, may not confer enough stability to produce consistently good results in the cat. Adding an intramedullary pin to the ulna in addition to the radial repair has been associated with more reliable surgical outcomes in this species.103 In addition, the ulna, especially its proximal portion, has been identified as a common site of nonunion in the cat, which may also be a significant contributory factor to surgical complication statistics for these fractures.84
Capital Femoral Physeal Fractures
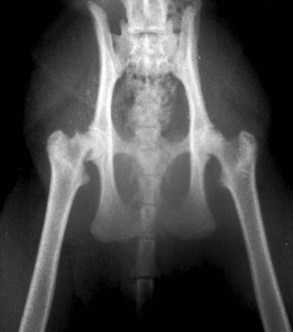
Capital femoral physeal fracture is a common traumatic injury in cats, but it appears as though just as many cases arise without a traumatic episode (Figure 26-6). Affected cats usually present with acute hind limb lameness, although the lameness may be mild and chronic in some instances. Most of these cases are seen in overweight, neutered males between 4 and 24 months of age.11,19,45,79 One report found a preponderance of domestic shorthairs,79 whereas another found a large number of Siamese cats.19
The first report of this problem described it as metaphyseal osteopathy of the femoral neck, which was thought to result from an aseptic necrosis, not unlike Legg-Calve-Perthes disease in the dog.88 However, further examination of serial radiography and histopathologic specimens suggests that the changes in the femoral neck are more likely to be resorptive and remodeling changes secondary to the Salter–Harris I physeal fracture rather than a vascular impairment as is seen in Legg-Calve-Perthes disease in the dog (Figure 26-7).19,45,79 The etiopathogenesis of this condition seems to revolve around abnormalities of the physis. Radiographically and histologically, these cats have abnormally wide physes that remain open long after they would be expected to have closed. Histologically, these physes are characterized by an irregular arrangement of chondrocytes rather than the normal columnar appearance, which has resulted in the use of the term physeal dysplasia to describe the process.
Although genetic factors may certainly be involved in the development of the physeal dysplasia, endocrine factors may also play a role. Neutering at an early age has been shown to delay physeal closure times in the cat,53 and it has been suggested that neutering before 6 months of age may be the endocrine factor contributing to the physeal dysplasia and slipped femoral capital physis.79 A similar syndrome occurs in young, overweight adolescent humans, especially those that are hypothyroid, are receiving growth hormone supplementation, or have hypogonadism.72 It may be that early neutering is one factor in the development of a physeal dysplasia, a wide physis, and a physis that remains open longer than normal, particularly in individuals that may be predisposed. If the cat becomes overweight, the stresses on the abnormal capital femoral physis may cause it to “slip,” producing the characteristic Salter–Harris I fracture. Published reports suggest that between 24% and 38% of affected cats will develop bilateral fractures.19,45,79,88 The condition is best treated with femoral head and neck excision (FHNE), which will produce a return to normal function in the majority of cases.45,79 Primary repair with Kirschner wires has been described20,29 but has much greater potential for complications than FHNE, with few, if any, demonstrated advantages.
Pelvic Fractures
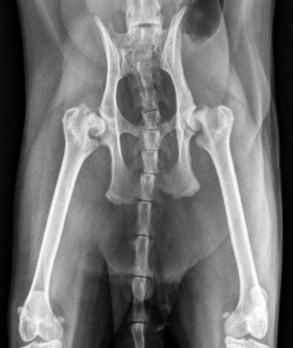
Pelvic fractures are extremely common in the cat, especially after vehicular trauma, and make up at least 22% of all fractures seen (Figure 26-8).64 Most pelvic fractures are multiple, unstable, and displaced, at least to some degree. Regardless, most will heal with conservative therapy. However, the issue is not whether they will heal but rather the severity and consequences of the malunion that almost invariably results. Although surgery can be considered to hasten pain relief and return to function, in most practical applications there are two primary indications for surgery:
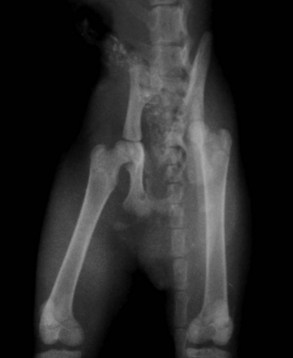
FIGURE 26-8 A kitten with multiple pelvic fractures. Note the significant decrease in pelvic canal diameter.
Displaced acetabular fractures: Approximately 17.5% of feline pelvic fractures involve the acetabulum. Any degree of fracture malunion in the coxofemoral joint will lead to degenerative joint disease (DJD) and pain. Such a fracture can be addressed near the time of the initial trauma by primary fixation methods that include plates, screws, and tension band wires.64 Traditionally, caudal acetabular fractures have often been treated conservatively because this area of the acetabulum was not considered to be weight bearing. However, recent research has suggested that the central and caudal portions of the acetabulum are actually the major weight-bearing regions within the coxofemoral joint of the cat.4 Alternatively, femoral head and neck ostectomy can be performed several days or weeks later, once the patient’s condition has stabilized, and where there is evidence of ongoing disability.
Pelvic canal narrowing: Ilial and acetabular fractures commonly displace axially, producing a narrowing of the pelvic canal. This can have immediate traumatic effects on bladder and bowels, but the greater concern is the prospect of producing obstipation and megacolon in the longer term. These problems are extremely frustrating to treat and are much better prevented. Thus pelvic fractures that produce more than 25% to 30% narrowing of the pelvic canal are best treated surgically. This can be efficiently done, in most cases utilizing bone plates, within 5 days of the initial trauma. After that time it becomes more difficult to break down fibrous tissue and surgically reduce these fractures. Pelvic canal–widening procedures such as pelvic symphyseal osteotomy can be performed if constipation has been present for less than 6 months. If constipation has been present for more than 6 months, the colon is often beyond reclamation and subtotal colectomy should be performed.17
Nonunion Fractures
Cats may be characterized as the “perfect orthopedic patient” in many ways because their straight bones, lightweight frames, and legendary healing abilities have resulted in many amazing outcomes in fracture cases. However, the old adage that fractured feline bones will heal if placed in the same room is not always true. Feline fracture nonunions do occur, at a rate of 4.3% according to one report.84 The tibia and proximal ulna were identified as the most common sites for nonunions. Increasing age and body weight, as well as open and comminuted fractures, were identified as risk factors.84
Arthridities
Degenerative Joint Disease
The slowly progressive degeneration of articular cartilage with osteophyte production, usually associated with acute or chronic joint trauma, is the most common form of joint disease seen in the cat and is variously described as DJD, osteoarthritis, or osteoarthrosis. It is only relatively recently that the common occurrence of DJD has been recognized in the cat. Knowledge about DJD in cats—prevalence, impact on lifestyle, efficacy of therapy—is less well developed than for the dog. Because cats have a small body size and are light and agile, they compensate for orthopedic diseases better than dogs. Cats are also notorious for hiding signs of illness, especially if onset is insidious, and it is more difficult to interpret signs of pain or discomfort in this species.100
Twenty-two percent of cats in a general population older than 1 year of age37 and 90% of cats older 12 years of age51 were found to have radiographic evidence of DJD. The elbow was the most frequently affected joint in the older population. The coxofemoral joint may also be affected, and most cats have bilateral involvement.15 Relatively few of these cats had clinical signs associated with the radiographic findings, or, perhaps more accurately, clinical signs were infrequently recognized by owners and veterinarians.16 This may be because the most common clinical sign associated with DJD in the dog is lameness. Owing to the cat’s lightweight frame and behavioral differences, it appears as though there may be other, more significant clinical signs of DJD that need to be recognized (Box 26-1). Physical examination findings for DJD in cats are also different than those for dogs (Box 26-2).
The hallmark of DJD is the progressive and permanent damage of articular cartilage.82 Injury of the chondrocytes leads to the production of inflammatory mediators such as cytokines (especially interleukin-1 [IL-1]) and tumor necrosis factor-alpha. IL-1 stimulates production of degradative enzymes, inhibits production of proteoglycans, and stimulates fibroplasia of the joint capsule. The thickened joint capsule contributes to stiffness and decreased range of movement.
When taking a medical history, especially for senior cats, the veterinarian should focus the questions on changes in activity and behavior rather than solely on lameness. Many signs of chronic pain are not obvious to owners or may be misinterpreted as due to aging.100 The degree of impairment caused by chronic pain may not be apparent to some owners until improvements occur after treatment.
Radiographic signs of DJD in cats are variable.1,16,36 Radiographs are best at demonstrating bony changes, and changes in the cartilage and synovium are not well demonstrated on plain radiographs. Joint effusions and joint capsule thickening are rarely evident. Typical bony changes include osteophyte development, subchondral sclerosis, perichondral bone erosion, and change in congruity of articular surfaces. Soft tissue swelling around the joint may be present. Lumbosacral DJD may be indicated by collapse of the L7-S1 disk space, sclerosis of the L7-S1 endplates, and spondylosis deformans.
Infectious Arthridities
Bacterial
Septic bacterial arthritis is most commonly associated with bite wounds sustained in cat fights. Hematogenous spread of bacteria from other sites in the body appears to be relatively rare in the cat, although it may occasionally be seen in kittens. Septic arthritis may develop secondary to orthopedic surgical procedures. Clinical signs include pain and swelling of the affected joint. Pyrexia and leukocytosis are usual but not invariable.56 Radiographic signs in the early stages will be confined to joint effusion and soft tissue swelling. As the condition progresses, and depending on the infective organism, there may be evidence of a periosteal reaction, bony sclerosis, and varying degrees of bone lysis at the periosteum and in the subchondral bone. Diagnosis is based on clinical signs and the results of arthrocentesis. Cytology, as well as aerobic and anaerobic culture combined with bacterial sensitivity testing, is essential, although a negative culture result is not uncommon. Therapy with bactericidal antibiotics (based on sensitivity testing) is indicated for 4 to 6 weeks. In the absence of a positive culture, while awaiting results, or where empirical treatment is desired, cephalosporin or amoxicillin–clavulanate antibiotics are reasonable choices, with metronidazole a useful addition in confirmed or suspected anaerobic infections.56 All of these antibiotic choices, and others besides, commonly cause vomiting or inappetence in cats, which may necessitate changes in therapy. Surgical drainage or flushing of infected joints is rarely necessary except in the most severe cases. Analgesics and other supportive care may be indicated.
Although up to 15% of cats have been found to be seropositive for Borrelia burgdorferi, they seem to be resistant to clinical disease, and no reports of arthritis associated with Lyme disease have been documented in this species.56
Mycoplasma
Rare cases of polyarthritis and tenosynovitis associated with Mycoplasma gateae and Mycoplasma felis have been reported in the literature.56,107 Hematogenous spread from areas of active or latent infection in respiratory mucous membranes or the urogenital tract, most often in otherwise debilitated or immunocompromised individuals, appears to be the pathogenesis of the arthritis. There is potential for diagnostic confusion with immune-mediated arthridities because Mycoplasma arthritis may appear similar. Radiographically, there is the potential for erosive lesions. Synovial fluid analysis and a negative aerobic culture may suggest immune-mediated arthritis. The organisms may be detected on a synovial fluid smear stained with Wright, Leishman, or Giemsa stains, or it may be grown on anaerobic culture from synovial fluid or synovium.56,107 The clinician should have an index of suspicion for Mycoplasma arthritis when dealing with debilitated individuals that appear to have immune-mediated arthritis. Tylosin, erythromycin, and gentamicin have traditionally been the most recommended therapies. However, fluoroquinolones represent a more recent, readily available alternative that is effective, convenient, and safe in cats.107
Viral
A short-term, self-limiting polyarthritis associated with calicivirus has been described in kittens younger than 6 months of age.7,21 The arthritis may be caused by infective intraarticular live virus or by the deposition of immune complexes within the synovium. The condition may be seen in association with the typical respiratory infection, or it may be seen 5 to 7 days after vaccination with modified-live calicivirus vaccine. Vaccine-associated arthritis is now uncommon because vaccine manufacturers have for the most part discontinued the use of virus strains associated with the problem. Diagnosis is made largely on the basis of history and clinical signs, and therapy is supportive, including analgesia, because the condition is self-limiting.7,21
Autoimmune Arthridities
Erosive
Periosteal Proliferative Polyarthritis
Periosteal proliferative polyarthritis (PPP) is the most common erosive form of arthritis seen in the cat. It most often affects the hocks and carpi of young adult male cats. Clinical signs begin acutely with fever, depression, stiffness, joint effusion, and pain. Over the course of a few weeks, the disease enters a chronic phase in which extensive periosteal new bone forms around the hocks and carpi and at the attachments of ligaments and tendons. New bone production may be extensive enough to produce ankylosis of joints. Erosive lesions may also be seen in subchondral bone and at tendinous attachments.21,56 Erosive lesions or periosteal new bone formation at attachment points of ligaments, tendons, or fascia are referred to as enthesopathies.7 The criteria proposed by Bennett and Nash for diagnosing PPP are found in Box 26-3.7
BOX 26-3 Criteria Proposed for the Diagnosis of Periosteal Proliferative Polyarthritis7
An etiologic link has been proposed between feline syncytium-forming virus (FeSFV) and PPP insofar as all cats with PPP appear to have FeSFV. However, attempts to experimentally induce PPP by inoculating cats with the virus have failed, and FeSFV has been found as a normal inhabitant in the joints of many asymptomatic cats.21 A role for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) has also been proposed in producing immunosuppression that allows proliferation of FeSFV; however, FeLV and FIV are frequently not found in cats with PPP.5,21 Occasionally symptoms of immune-mediated arthritis may be found in cats immunocompromised for other reasons, including chemotherapy and hyperadrenocorticism.
PPP bears some similarities to Reiter’s disease, which is seen most commonly in men. Urethritis and diarrhea can be seen, in addition to the arthritic lesions in humans, and there is at least one report of hematuria associated with polyarthritis in a cat.5 Conjunctivitis and lesions of skin and mucous membranes, which are common in humans, have been found in some cats. In light of these types of lesions, a link between Reiter’s disease and Chlamydia infection has been explored, and the organism has been implicated in humans.7 The prognosis for cats with PPP is guarded to poor. Few will experience anything more than marginal improvement on therapy, and many will end up being euthanized. However, decisions of that sort should be based on assessments of the patient’s function and comfort level.
Rheumatoid Arthritis
By all accounts, rheumatoid arthritis (RA) is much less common in the cat than in the dog. RA is a synovitis caused by the production of autoantibodies against immunoglobulin G (rheumatoid factor). The deposition of immune complexes within the synovium leads to an erosive, deforming arthritis. As with other immune-mediated arthridities, RA causes generalized stiffness, pain, and swelling of joints. In contrast to PPP and idiopathic immune-mediated arthridities, there is seldom malaise or inappetence, and the course tends to be more gradual in onset. Joint deformity to the point of subluxation and luxation is often a prominent feature. Bennett7,21 has described 11 diagnostic criteria, patterned after those used in man and the dog, for establishing a diagnosis of RA in the cat (Box 26-4). The criteria recognize the fact that a positive blood test for rheumatoid factor is not always present, and a positive test is not necessarily specific for the disease. The presence of subcutaneous nodules is common in humans but has not been described in the cat.7
BOX 26-4 Diagnostic Criteria for Establishing a Diagnosis of Rheumatoid Arthritis in the Cat7
2 Pain on manipulation of at least one joint
3 Swelling of at least one joint
4 Swelling of at least one other joint within a 2-month period
7 Erosive radiographic changes
8 Positive rheumatoid factor blood test
9 Abnormal synovial fluid (poor mucin clot, predominance of polymorphonuclear cells)
10 Characteristic histologic changes in the synovium (villous hypertrophy)
11 Characteristic histologic changes in the subcutaneous nodules
One report of 12 cases of RA in the cat described an average age of 5.9 years, with Siamese cats overrepresented.44 With aggressive forms of antirheumatic therapy, discussed at the end of this section, the prognosis for RA seems to be much better than PPP, with 58% showing a marked improvement.44
Non-Erosive
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an uncommon cause of arthritis in cats. It is characterized by multisystemic involvement with polyarthritis and usually one of the following: autoimmune hematologic disease (thrombocytopenia, hemolytic anemia, and leukopenia), dermatitis, glomerulonephritis, or meningitis.7 There are no destructive or deforming lesions on radiography or gross examination. The disease results from an autoimmune reaction to nucleic acid, which manifests as the hemolytic anemia, leukopenia, or thrombocytopenia. Deposition of the resulting immune complexes produces synovitis, glomerulonephritis, meningitis, dermatitis, or occasionally polymyositis. Onset of symptoms is usually acute and includes pain, especially on handling, and joint swelling.
Clinical pathology is essential to diagnose SLE. Antinuclear antibody (ANA) blood tests are invariably positive at a level of 1 : 40 or greater in the cat.7 Although a diagnosis of SLE cannot be established without such a positive ANA value, the test is not specific and may be elevated in the acute phase of other disease conditions, including FeLV, FIV, and feline infectious peritonitis (FIP). Moreover, lower titers of ANA can be found in otherwise healthy cats.7 Hematologic abnormalities (anemia, thrombocytopenia, and leukopenia) are common, as is proteinuria.7 The diagnostic criteria to establish SLE are as follows:
1 Multisystem involvement, which most often includes polyarthritis and one other body system
2 ANA titre of 1 : 40 or greater
3 Presence of antibodies to blood cells or immune complexes in histopathology of affected tissues
Criteria 1 and 2 are essential for the diagnosis, which cannot be made solely on the basis of an elevated ANA titer.7 Reports of treatment for SLE in cats are rare in the literature, but the prognosis appears to be guarded.
Idiopathic Polyarthritis
Polyarthridities that do not fit into any of the other classifications end up in the idiopathic category. There are four subgroups of idiopathic polyarthritis (Box 26-5).7
BOX 26-5 Idiopathic Polyarthritis Subgroups7
Type II: idiopathic associated with other infections. These may be found in the respiratory or urogenital tracts, skin, or oral cavity.
Type III: idiopathic associated with gastrointestinal disease
Type IV: idiopathic associated with neoplasia. In the cat this is most often myeloproliferative neoplasia, which may be FeLV or FIV.
Idiopathic arthritis may present at any age, but most affected cats are young adults that develop symptoms acutely or subacutely. The pathogenesis is undoubtedly a synovitis arising from the deposition of immune complexes. Clinical signs are acute in all but type IV cases, which have a more chronic course. Most cases of all types exhibit signs of stiffness and pain, with occasional lameness. Joint and soft tissue swelling is usually present. These changes, along with the clinical signs, tend to be bilaterally symmetric. Pyrexia and inappetence are common. Type II idiopathic polyarthritis in the cat is most often associated with respiratory symptoms, including increased lung sounds, congestion, and conjunctivitis. Cats with type III frequently have diarrhea and dysentery, with occasional vomiting.7 Toxoplasmosis has been identified as one possible cause.7 Radiography is normal, except for joint effusion in some cases. The prognosis with treatment is generally good, especially in type I cases or in type II and III cases in which the underlying systemic illness can be identified and treated. Relapses, especially in types I and IV, are common.
Scottish Fold Arthropathy
Scottish Fold cats have an autosomal dominant trait that impairs enchondral ossification and produces abnormal cartilage maturation called Scottish Fold arthropathy (also known as osteochondrodysplasia and osteodystrophy). Heterozygotes for this trait have the characteristic “folded ear” appearance for the breed and may have mild signs of arthropathy. Homozygotes develop a progressive ankylosing arthropathy with radiographic lesions evident as early as 7 weeks of age. The condition is characterized by the production of new bone that bridges joints throughout the body but most prominently in the paws, spine, tail, and distal hind limbs (Figure 26-9). Affected individuals have a short, squat appearance that has been described as a form of dwarfism14 and experience decreased range of motion in affected joints. Clinical signs include stiffness, lameness, and inability to jump. The condition is progressive and can produce dramatic periarticular new bone, most prominently on the plantar aspect of the calcaneal–tarsal–metatarsal articulation. Exostoses in this area can produce ulceration of overlying skin. Surgical excision of the exostoses or radiation therapy have been used as successful palliative therapies for extended periods.14,54,78 Nonsteroidal antiinflammatory drugs (NSAIDs), pentosan polysulfate, and oral glycosaminoglycans have been reported to provide symptomatic relief.14,74 One report has described the use of pantarsal arthrodesis to improve function.78
Arthritis Therapy
Therapy for Degenerative Joint Disease
Drug therapy is indicated to control inflammation, provide pain relief, and improve function. The development of several NSAIDs specifically for the small animal veterinary market is arguably one of the most significant therapeutic advances in veterinary medicine during the last 50 years. Despite this, relatively little information exists regarding the safety and efficacy of these drugs in the cat, and virtually nothing is known about the long-term use of these drugs in this species for chronic pain and inflammation, such as is seen in DJD. What is known is that the cat has a significantly decreased ability to metabolize most of these drugs through hepatic glucuronidation. The result is a much longer half-life for most NSAIDs in the cat and thus a greater potential for accumulation and toxicity. Whereas gastrointestinal upset characterized by vomiting, inappetence, and diarrhea is the most common toxic effect, renal insufficiency is the most serious potential adverse reaction. Both of these adverse reactions arise from the antiprostaglandin effect of NSAIDs, specifically on the cyclooxygenase 1 (COX-1) isomer in the inflammatory pathway. Prostaglandins have a protective effect on gastric mucosa, presumably through increased secretion of mucus in the gastrointestinal tract. Inhibition of this secretion, combined with the acidic nature of most NSAIDs, produces gastric inflammation and ulceration. In the kidney, prostaglandins play a role in maintaining renal blood flow, particularly in the face of dehydration or hypotension. Inhibition of this effect has been associated with catastrophic renal failure in some cats.13,67,91 The potential for these adverse reactions undoubtedly explains some of the reluctance on the part of pharmaceutical manufacturers to pursue the use of NSAIDs in this species. Nevertheless, limited research has resulted in label recommendations in some parts of the world for feline use of carprofen, ketoprofen, tolfenamic acid, and meloxicam.13,43,67,80,91 Only meloxicam has a label for extended duration of administration in some countries, but cautious off-label use of various NSAIDs has been successful for much longer periods.43,91,99a
Carprofen is labeled for use in the cat in the United Kingdom, Europe, Australia, and New Zealand; however, the indication is for one-time postsurgical subcutaneous administration at a dose of 4 mg/kg.67,80,91 No information exists on long-term use other than an individual report of toxicity after oral administration.67 The drug has an extremely variable half-life (9 to 49 hours) in the cat, which increases the likelihood for toxicity with repeated use in some cats. Consequently, carprofen cannot be recommended as a safe option for management of DJD.
Ketoprofen is approved for use in the cat in Europe, Australia, and Canada at a dose of 1 mg/kg orally, once daily for up to 5 days, and 2 mg/kg subcutaneously, once daily for up to 3 days. Although the drug is eliminated by hepatic glucuronidation in the dog, the half-lives in both dogs and cats are similar. This, combined with other evidence, suggests the possibility of another excretory pathway for ketoprofen in the cat, which may imply a higher degree of safety; however, no long-term data corroborate this.67 The drug is known to have a relatively greater activity against COX-1, which increases its inhibitory effect on platelet aggregation and may be significant in terms of renal or gastrointestinal side effects with long-term use.67
Tolfenamic acid is licensed for use in cats in Canada, Australia, New Zealand, and most of Europe. The recommended dose is 4 mg/kg orally or subcutaneously, once daily for 3 to 5 days. The product is labeled for the treatment of fever and upper respiratory disease, although its use as an analgesic and antiinflammatory is established in the literature.67 Information provided by the manufacturer claimed no significant toxic effects when up to twice the recommended dose of the oral tablet or the injectable solution were given for up to 10 days to two groups of 12 cats. Increases characterized as “mild” were noted in the alanine transaminase (ALT) and aspartate aminotransferase (AST) of treated cats, and a positive test for fecal occult blood was noted in two cats from each group. There are anecdotal reports of DJD treatment in cats with tolfenamic acid for 3 to 5 consecutive days each week for extended periods, but little information in the literature supports this recommendation.
Most of what little information exists regarding the chronic use of NSAIDs for DJD in the cat concerns meloxicam. In contrast to the metabolism of most other NSAIDs in the cat, meloxicam is excreted by oxidative enzymes and thus has a similar half-life in both the cat and dog.67 One recent study showed a COX-1–sparing effect for meloxicam in cats.38 Consequently, the drug lends itself more readily to longer-term use in this species. In North America meloxicam is supplied in a 1.5 mg/mL honey-flavored oral formulation that is well tolerated by cats. In many countries it is supplied in a cat-specific formulation of 0.5 mg/mL. The same strength is available in the United States as a small dog formulation. It is labeled for feline use in the United States, Europe, Australia and New Zealand at a dose of 0.3 mg/kg for one-time subcutaneous use. Further, chronic use of the oral solution at 0.05 mg/kg per day is described on the package insert in many countries.91 Several other recommendations for oral dosing appear in the literature (Box 26-6).
BOX 26-6 Published Doses for Meloxicam in the Cat
| Source | Dose |
|---|---|
| Label: US, Australia, New Zealand, EU | 0.3 mg/kg subcutaneously, once |
| Label: Australia, New Zealand, EU | 0.1 mg/kg on day one, followed by 0.05 mg/kg per day, orally |
| Wallace 2003,103a Carroll & Simonson 200512a | 0.2 mg/kg on day 1, followed by 0.1 mg/kg/day for 2 days, then 0.025 mg/kg/day or 0.1 mg/cat 2-3 times/week |
| Lascelles 200767 | 0.1 mg/kg on day 1, followed by 0.05 mg/kg for 1 to 4 days, then reduction to the lowest effective dose (0.025 mg/kg every 24-48 hours) |
| Robertson 200891 | 0.05 mg/kg given once, followed by 0.025 mg/kg or less once daily |
| Gunew 200843 | 0.1 mg/kg for four days, followed by 0.1 mg/cat once daily |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree