• Weakness, muscle fasciculations and collapse • Generalized muscle weakness of neurological origin (e.g. equine degenerative myeloencephalopathy) or neuromuscular origin (e.g. botulism) • Encephalitis (e.g. West Nile virus infection) (can cause muscle fasiculations) • Electrolyte disorders (e.g. hypocalcemia) • Obstruction of the respiratory tract (e.g. foreign body) • Pharyngeal/laryngeal paralysis (e.g. secondary to nerve damage in strangles cases). • Definitive diagnosis is by genetic testing. This can be performed on whole blood or hair root. The American Quarter Horse Association (AQHA) will accept HYPP test results only if performed through a licensed laboratory. Testing kits for non-registration purposes are available from the AQHA. • Diagnosed subjectively by clinical signs and signalment. • Post-mortem samples that can be collected if a horse has died during a suspected attack are: hair samples for DNA testing and aqueous humor for potassium concentration. • Mild cases can be treated with light exercise, by feeding a readily absorbable source of carbohydrate (oats), and/or with acetazolamide (3 mg/kg orally). • For severe cases, the following treatment is recommended: intravenous administration of 5% dextrose with sodium bicarbonate (1–2 mEq/kg) and intravenous administration of 23% calcium gluconate (0.2–0.4 ml/kg ) diluted in 5% dextrose (4.4–6.6 ml/kg). • Management is an important feature of control and should include regular exercise and ideally frequent paddock turnout. Wholegrain feeds should be fed instead of sweet feed. Avoid feeds with high potassium content such as alfalfa and replace with timothy or Bermuda grass hay. Give several small feeds daily at regular intervals. • Avoid stressors such as rapid changes in diet, exposure to cold or overexertion. • Acetazolamide (2–4 mg/kg q 8–12 h) has been recommended to decrease the frequency and severity of episodes. The daily dose can be decreased over time until the lowest effective dose is reached. • Frequently a tentative diagnosis can be made on the basis of breed, age and clinical signs. • Definitive diagnosis requires EMG examination. The pathognomonic finding is described as crescendo-decresendo, high-frequency repetitive bursts with a characteristic ‘dive-bomber’ sound. • Muscle biopsies are not useful for diagnosis as they may be normal. • The most accurate form of diagnosis is through genetic testing but there is currently limited availability. • Other findings are not consistent but include: • Basophilic globules and eosinophilic crystalline material in hematoxylin and eosin stains • Periodic acid-Schiff (PAS) staining of cardiac and muscle sections may contain PAS-positive globular inclusions. • There is no effective treatment and therefore early identification and euthanasia are is essential to avoid unnecessary costs in neonatal care untis. The clinical signs seen are somewhat breed-dependent: • Quarterhorses. The most common signs are firm, painful muscles, stiffness, fasciculations, weakness, sweating and reluctance to move. The hindquarters are most commonly affected but other muscles can also be involved. Signs of pain can be severe and long-lasting. Signs can be seen with minimal work especially in horses that have been rested for several days or are on a high-grain diet. There is no specific association to gender, body type or temperament. Average age of onset of clinical signs is 5 years but signs have been seen in horses as young as 1 year of age. Serum CK and AST activities are often persistently high in affected horses with median values reported at 2 809 and 1 792 U/L, respectively. • Draft horses. Many affected horses do not have clinical signs. Rhabdomyolysis and myoglobinuria can be seen in horses that are fed high-grain diets, exercised irregularly or have little turnout. Signs can also be seen with general anesthesia. Again there is no gender predilection and the mean age of onset of clinical signs is 8 years. The median CK and AST levels in affected horses are reported to be 459 and 537 U/L, respectively. • Warmbloods. Warmbloods may have either type 1 or type 2 PSSM depending on the crosses from which they are derived. The most common clinical signs noted are painful, firm back and hindquarter muscles. Overt rhabdomyolysis is not that commonly seen. The mean age of onset is 8–11 years of age and the median CK and AST activities are 323 and 331 U/L, respectively. • Type 1 PSSM can be diagnosed through genetic testing which is available through the University of Minnesota. Muscle biopsies can also be used with the characteristic findings of amylase-resistant granular polysaccharide in amylase periodic acid-Schiff (PAS) stains. • There is no genetic test available for type 2 and muscle biopsies demonstrate increased or abnormal PAS-positive material that is usually amylase-sensitive. • However, because the diagnosis of PSSM based on muscle biopsies alone is quite subjective it is important that the horse is thoroughly evaluated to rule out other conditions. • Horses with PSSM should ideally have some form of turnout available. • Exercise regimens should only be implemented after there has been adequate time allowed for dietary changes (2 weeks). The duration and not just the intensity of exercise is important and exercise should be gradually introduced and consistently performed. It is also important to minimize the number of days without some form of exercise. • Dietary management is very important in affected horses and should first consist of reaching the ideal body weight for the horse. Once this has been done the dietary modifications should combine reducing glucose load and providing fat as an alternative energy source. There are now a wide variety of commercially produced diets available for horses with PSSM. The basic dietary principle is that less than 10% of digestible energy (DE) be provided in the form of starch and at least 13% in the form of fat. • Evaluation of serum CK and AST levels is used by many to identify poor performance in association with subclinical episodes of RER. In doing such tests it is important to be consistent with regard to sampling times. The ideal is 4–6 hours after exercise but in many cases due to yard management this is not possible and then samples should be routinely collected at a standardized time in relation to the previous day’s work. • If serum muscle enzymes are normal but RER is still believed to be a cause of poor performance an exercise test can be performed with samples taken before and 4–6 hours after exercise. Exercise for such tests should consist of 15 minutes of collected trotting. A 3–4-fold rise in serum ck activity is indicative of subclinical muscle damage. • While there is currently no genetic testing available for RER horses it is an area of active research and such a test may be available in the near future. • The goals of therapy for specific episodes of exertional rhabdomyolysis are relieving pain and anxiety, replacing fluid and electrolyte losses and maintaining renal function. • Non-steroidal anti-inflammatories are frequently used to relieve pain and in mild cases may be the only treatment necessary. However, they should be cautious or combined with intravenous fluid therapy in dehydrated horses. • Severely affected horses, dehydrated horses or horses with myoglobinuria should receive intravenous fluids. Hyperkalemia can occur in some horses and can be treated by the administration of isotonic sodium chloride. Some horses will be hypocalcemic and will require supplementation with calcium. • Other drug therapies include dantrium sodium (4 mg/kg PO q 4–6 h) which decreases muscle contracture and may help prevent further muscle necrosis. Methocarbamol (a muscle relaxant) (5–22 mg/kg IV slowly) produces variable results perhaps as a reflection of the dose used. Corticosteroids are advocated by some especially if the horse is recumbent. DMSO has been used as an anti-oxidant, anti-inflammatory and osmotic diuretic. Tranquilizers may help relieve anxiety and the perpetuation of an episode.
Muscle
Congenital/developmental disorders
Hyperkalemic periodic paralysis (HYPP) (Fig. 8.1)


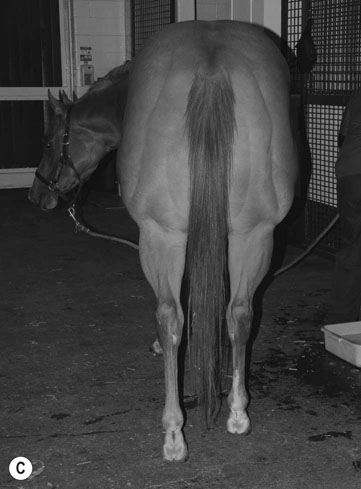
Differential diagnosis:
Diagnosis and treatment
Control
Myotonia congenita (Fig. 8.2)
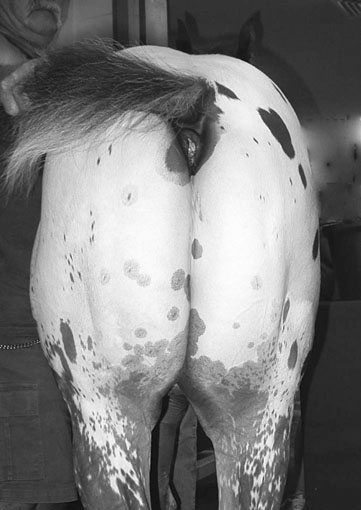
Differential diagnosis:
Diagnosis
Glycogen branching enzyme deficiency
Diagnosis and treatment
Polysaccharide storage myopathy (PSSM) (Figs. 8.3 & 8.4)
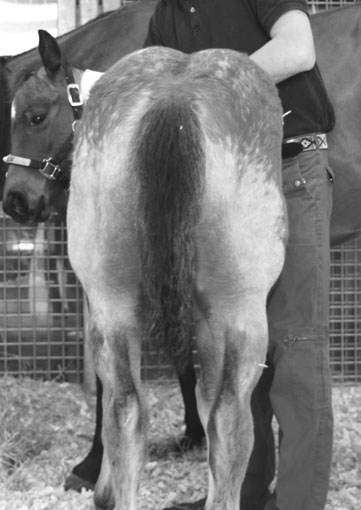
Diagnosis and treatment
Management
Recurrent exertional rhabdomyolysis (RER)
Diagnosis
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



