Chapter 4 Mouse
Introduction
A detailed presentation of the embryology, anatomy and histology of all the different organ systems discussed is beyond the scope of the current chapter, and the reader is referred to relevant texts for each of the different organs discussed. A general review of the anatomy and biology of the laboratory mouse and the use of mice in biomedical research has been presented in various publications (Cook 1965, Green 1966, Hedrich & Bullock 2004, Hummel et al 1966, Komárek 2004, Krinke 2004, Percy & Barthold 2007, Staats 1966).
Many sources of information on the spontaneous, non-neoplastic pathology of laboratory mice are available. The aging patterns of mice and strain variations in background pathology have been reported (Brayton 2007, Cotchin & Roe 1967, Hedrich & Bullock 2004, Mohr et al 1996a, b, Russell 1966, Russell & Meier 1966). The pathology of aging mice based on the studies performed at different laboratories has been presented for the BALB/c and B6C3F1 mouse (Frith et al 1985, Frith & Ward 1988), the CD-1 mouse (Faccini et al 1990), and those strains of mouse used in the generation of genetically engineered mice (Haines et al 2001, Mahler et al 1996, Percy & Barthold 2007, Ward et al 2000). Descriptions of spontaneous, murine, non-neoplastic pathology are also included in other publications reviewing the spontaneous and induced lesions in different organ systems in rodents in pre-clinical toxicity and carcinogenicity studies (Gad 2007, Jones et al 1983, Jones et al 1985a, b, Jones et al 1986, 1988, 1991, Maronpot et al 1999, Mohr 2001).
The National Toxicology Program (NTP) routinely uses the B6C3F1 mouse, an F1 hybrid of two inbred strains, the C57BL/6 and C3H strains (Rao & Boorman 1999), in their toxicity and carcinogenicity studies. The outbred CD-1 mouse is also routinely used throughout the world in pre-clinical testing of pharmaceuticals, agrochemicals and industrial chemicals (Gad 2007).
The NTP publishes on their web sites the observations recorded in the numerous toxicity and carcinogenicity studies performed by them every year, and maintain a historical control database of neoplastic findings classified by species, strain, route of administration, vehicle and diet (http://ntp.niehs.nih.gov/).
Animal suppliers also routinely maintain historical control pathology data, and this information can usually be retrieved from their web sites (Charles River: http://www.criver.com/; Harlan: http://www.harlan.com/) although this may be limited to neoplastic findings for some strains of animals.
There is a need to maintain an up-to-date source of information on the ever increasing numbers of inbred and genetically modified strains of mice used in biomedical research and those differences in biology associated with these new genotypes (Anagnostopoulos et al 2001, Bolon 2007, Brayton et al 2001, Linder 2003, 2006, Linder & Davisson 2004, Yoshiki & Moriwaki 2006). The Jackson Laboratory maintains the Mouse Genome Informatics web site, a database designed to act as an international resource for the laboratory mouse, integrating genetic, genomic and biological data (http://www.informatics.jax.org/). This site offers resources and links to multiple databases, many of which provide valuable information for the toxicological pathologist involved in the evaluation of pre-clinical studies in the mouse. Other internet sources of information on genetically engineered rodents have been reviewed by Bolon (2006) and Linder & Davisson (2004).
Cardiovascular system
The embryology, anatomy, histology and physiology of the heart and blood vessels are reviewed by Elwell & Mahler (1999) and Michael et al (2004) and an extensive review of myocardial diseases of animals has been presented by Van Vleet & Ferrans (1986).
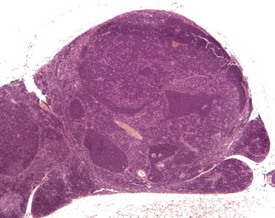
Congenital lesions in the heart are rare (Hagiwara et al 1996). Cardiomyopathy is a diagnostic term used to describe a spectrum of spontaneous, age-related, degenerative changes, including degeneration, necrosis and increased interstitial fibrous tissue. The inflammatory component of these changes varies (Berdanier 2004, Elwell & Mahler 1999, Frith et al 2007). The reported incidence of this finding is considered to vary partly because of variation in reporting levels and terminology between pathologists (Elwell & Mahler 1999). These changes can occur at an early age, and are occasionally responsible for the early death of mice on long-term studies (Son 2003a) (Fig. 4.1).
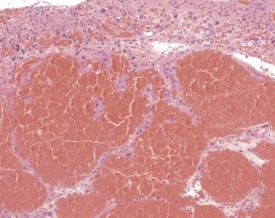
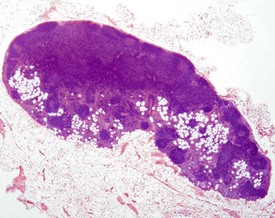
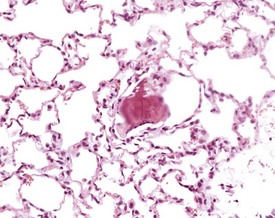
Atrial thrombosis is described as an uncommon lesion by some authors (Frith & Ward 1988, Frith et al 2007), but as common by others (Berdanier 2004, Faccini et al 1990). This probably reflects the strain and stock differences in the occurrence of this lesion. It does occur as a spontaneous lesion, however, in CD-1 mice, more commonly in the left atrium than in the right (Carlton & Engelhardt 1991, Elwell & Mahler 1999, Frith & Ward 1988, Frith et al 2007, Hagiwara et al 1996, Meier & Hoag 1966, Percy & Barthold 2007, Van Vleet & Ferrans 1986). The distended atrium contains an organizing, mural thrombus which may contain areas of cartilaginous metaplasia. The character and features of the thrombus are dependent on the age of the thrombus. There are strain and sex differences in the occurrence of atrial thrombi. Diet and parity also influence the occurrence. Atrial thrombosis was identified as one of the major causes of death or morbidity in a review of 11 long-term studies in CD-1 mice (Maita et al 1988) (Fig. 4.2).
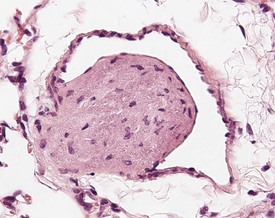
Dystrophic mineralization (dystrophic calcification) is a strain-specific finding and occurs at a high incidence and at an early age in susceptible strains. There are sex differences in the occurrence of this finding within strains, and parity and diet also influence its occurrence (Berdanier 2004, Eaton et al 1978, Elwell & Mahler 1999, Frith & Ward 1988, Frith et al 2007, Hagiwara et al 1996, Maeda et al 1986, Meier & Hoag 1966, Percy & Barthold 2007, Russell 1966, Van Vleet & Ferrans 1986, 1991a, Yamate et al 1987). The distribution of the mineralization is also strain specific and can be epicardial, myocardial or both. Myocardial mineralization is an uncommon finding in CD-1 mice (Faccini et al 1990), but is common in BALB/c mice (Fig. 4.3).
In old mice, cartilage cells can sometimes be observed in the fibrous tissue at the base of the heart valves (Hummel et al 1966). Endocardial myxomatous change has been described as an age-related lesion involving the generation of excess myxomatous matrix within valvular interstitial cells. This change is not associated with increased morbidity or mortality. The low level of reporting of this lesion is considered to be related to inconsistency of sampling in routine toxicity studies (Donnelly 2008, Elangbam et al 2002).
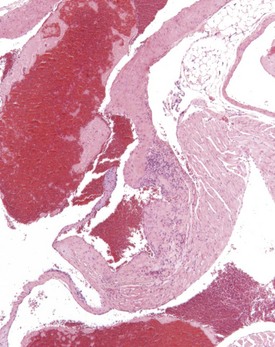
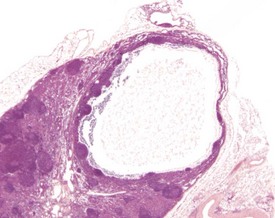
Perivascular lymphoid infiltrates frequently occur in older mice in salivary glands, kidneys and other organs (Percy & Barthold 2007). Inflammatory changes in the vasculature can occur as isolated lesions, affecting the vessels of a single organ, or can affect several systemic arteries. The occurrence and severity of this change varies with age, strain and sex, but the etiology is generally considered to be unknown (Elwell & Mahler 1999, Faccini et al 1990, Frith & Ward 1988, Frith et al 2007, Percy & Barthold 2007). There are reports of correlations between elevated blood pressure in mice and an increased incidence of polyarteritis (Mullink & Haneveld 1979). Histologically, the affected arteries typically show hyaline degeneration of the walls of the vessel with associated inflammatory cell infiltration affecting the intima, media and adventitia. Aneurismal dilatation and hemorrhage can be seen in more severe cases (Fig. 4.4).
Inflammatory changes involving the root of the aorta (aortitis) have been described in BALB/c mice (Ramot et al 2009). These authors considered that these changes have been under-reported because of variation in sampling of this area of the heart in routine toxicity studies (Fig. 4.5). Systemic polyarteritis has been reported as one of the major causes of death or morbidity in a review of 11 long-term studies in CD-1 mice (Maita et al 1988). Angiectasis, the focal dilatation of vascular channels, can occur in any organ, but usually affects the liver, spleen and lymph nodes of mice. The existing vascular spaces are dilated and prominent, and lining endothelial cells are normal in appearance, number and size. The etiology of this change is unknown (Elwell & Mahler 1999, Faccini et al 1990, Frith & Ward 1988, Frith et al 2007, Plendi et al 1996) (Fig. 4.6).
Hemolymphoreticular system
The embryology, anatomy, histology and physiology of the different elements of the hematopoietic system have been reviewed by a number of authors (Cesta 2006a, b, Pearse 2006a, Travlos 2006a, van Rees et al 1996, Ward et al 1999, Willard-Mack 2006). In addition, a recent monograph published by the Society of Toxicologic Pathology presented a number of papers covering the normal structure, function, pathology and enhanced histopathology of the hematopoietic tissues (Maronpot 2006).
The organs of the hematopoietic system represent a complex and dynamic system, where changes can occur in a single component of the system or be manifested by changes in all of the different organs. There is also a large variation in the normal appearance of the different tissues, dependent on the age and sex of the animals examined, as well as differences in the individual animal’s background of antigenic stimulation (Frith et al 1985, Wijnands et al 1996).
A limited number of lymph nodes are routinely examined in pre-clinical toxicity and carcinogenicity studies, with additional nodes usually being presented for examination because of abnormalities reported at necropsy. Accurate identification and naming of all the lymph nodes of the mouse is important to ensure that comparisons between studies relating to particular anatomical sites can be correctly made. An investigation into the normal distribution of lymph nodes in the mouse, with suggested standardized nomenclature, has been published by Van den Broeck et al (2006).
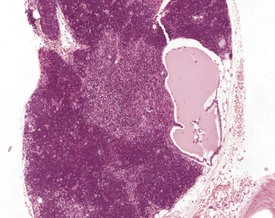
Sinus ectasia (lymphangiectasia, lymphatic cysts, sinus dilatation) is typically associated with lymphoid atrophy and can involve the medullary and subcapsular sinuses. It is found commonly in the mesenteric lymph nodes of aging mice. Dilated sinuses are lined by endothelium and can contain pale eosinophilic/amphophilic material. Ectasia is probably related to obstruction of efferent lymph vessels (Elmore 2006, Wijnands et al 1996) (Fig. 4.7).
Angiectasis is most often seen in the mesenteric lymph nodes. The significance of this change is unknown (Elmore 2006, Frith et al 1985, Ward et al 1999). Sinus erythrocytosis can result from a lymph node draining an area of hemorrhage, but this lesion can also be artifactual and related to handling procedures at necropsy. Depending on the duration of the initiating lesion, sinus erythrocytosis may be accompanied by hemosiderin-laden macrophages, erythrophagocytosis and inflammatory cells (Elmore 2006, Frith et al 1985, Wijnands et al 1996) (Fig. 4.8).
Sinus histiocytosis is characterized by accumulation of histiocytes in the subcapsular and medullary sinuses. The histiocytes have a characteristic eosinophilic cytoplasm and may contain pigment or other phagocytosed material (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2001, 2007). Pigment accumulation in sinusoidal macrophages is a common occurrence in aging mice. The pigments are most often hemosiderin or lipofuscin. Hemosiderin is an iron-containing, golden-brown, granular pigment, and is usually associated with sinus erythrocytosis. Lipofuscin is a golden-brown pigment derived from the breakdown of lipids from cell membranes and organelles (Berdanier 2004, Elmore 2006, Wijnands et al 1996) (Fig. 4.8).
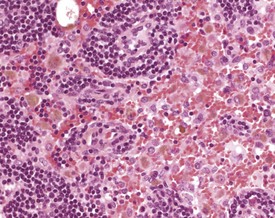
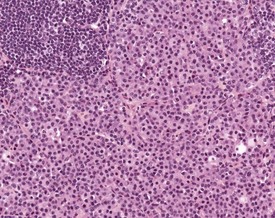
Lymphocyte hyperplasia may be evident to a certain degree in various lymph nodes, and can be dependent on the location of the lymph node and health status of the animal. These changes increase in incidence with age and occur more often in females than in males (Elmore 2006, Frith & Ward 1988, Frith et al 2001, Ward et al 1999, Wijnands et al 1996). Hyperplasia can involve different types of cell, most commonly plasma cells, but can include an increase in the number of mast cells.
Plasma cell hyperplasia (plasmacytosis) is a common finding in rodents, particularly in the submandibular lymph nodes, and is usually in response to antigenic stimulation (Elmore 2006, Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2001, 2007, Hummel et al 1966, Ward et al 1999, Wijnands et al 1996) (Fig. 4.9). Mastocytosis is an increase in the number of mast cells within the sinuses of the lymph nodes. Normal background levels of mast cells in the lymph nodes may vary by strain (Frith & Ward 1988, Frith et al 1985, 2001, 2007).
Atrophy or lipomatosis occurs in older mice. Adipocytes can often be seen in the hilus region or medulla of the lymph nodes of old mice (Ward et al 1999, Wijnands et al 1996) (Fig. 4.10). The spleen is the principal site for extramedullary hemopoiesis, but this change can sometimes be present in the lymph nodes of mice, in response to a physiological need such as hemorrhage or severe inflammation (Elmore 2006, Faccini et al 1990, Frith et al 2001).
Hyperplasia of dendritic reticular cells (interdigitating cells) is occasionally seen in young and old mice. These are seen in response to viral infections and other processes (Frith et al 1985, Tew et al 1982); they can occur in a single lymph node or affect multiple sites, and can occur as focal lesions in the paracortex. More extensive lesions involve the entire lymph node (Fig. 4.11).
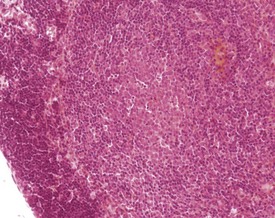
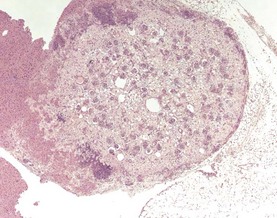
Accessory splenic tissue can be found occasionally, either in the pancreas or abdominal adipose tissue (Frith & Ward 1988, Frith et al 1985, 2007, Krinke 2004, Hummel et al 1966, Ward et al 1999). Extramedullary hemopoiesis (EMH) is a normal finding in the red pulp of the spleen in mice, and is usually more prevalent in young animals and females (Cesta 2006a, Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2001, 2007, Hardy 1967, Krinke 2004, Percy & Barthold 2007, Suttie 2006, van Rees et al 1996, Ward et al 1999, Wijnands et al 1996). Extramedullary hemopoiesis can consist of erythroid precursors, myeloid precursors, megakaryocytes or all three. The predominant feature of EMH depends on the initiating stimulus (the erythroid component predominates in response to hemorrhage, the myeloid component predominates in response to inflammatory conditions) (Suttie 2006). Increased EMH can often be seen in response to skin ulceration, abscesses, or the presence of large tumors (Fig. 4.12).
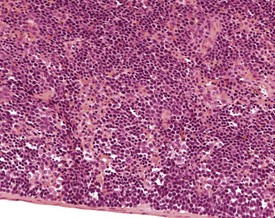
FIGURE 4.12 Extramedullary hemopoiesis in the red pulp of the spleen of a 20-week-old, female, CD-1 mouse. ×200.
Atrophy can affect the red pulp or the white pulp and may occur spontaneously in older mice (Faccini et al 1990, Suttie 2006, Ward et al 1999). Pigment accumulation is a common background lesion in the murine spleen. The pigment, which is usually hemosiderin, is contained in macrophages in the red pulp, but can also be present in the white pulp, and can be observed to accumulate in the spleens of older mice. It is more common in females than in males. In pigmented mouse strains, pigmentation of the spleen can occur as a result of accumulation of melanin (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2007, Hardy 1967, Percy & Barthold 2007, Suttie 2006, van der Heijden et al 1995, Ward et al 1999, Wijnands et al 1996).
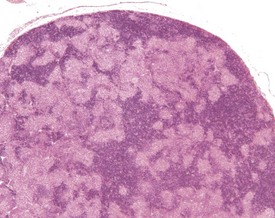
Hyperplasia of the splenic white pulp can increase in incidence with age. The lymphoid component of the spleen can increase in number and size, and enlarged follicles often coalesce (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2001, 2007, Ward et al 1999, Wijnands et al 1996).
Lymphoid hyperplasia of the Peyer’s patches, or other sites of mucosa associated lymphoid tissue (MALT) in the small and large intestine, may occur following antigenic stimulation (Faccini et al 1990, Maekawa et al 1996b, Shackelford & Elwell 1999). The term is usually reserved for a prominent increase in lymphoid tissue in an area where MALT is not normally conspicuous (Shackelford & Elwell 1999).
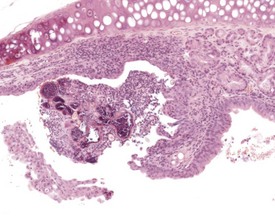
The thymus is larger in young animals compared to older ones, and reaches maximum size around the time of sexual maturity. The thymus is usually larger in females than in males. The normal, age-associated decrease in the cellularity of the thymus is termed involution, whereas reductions in cellularity associated with stress or toxicity are usually termed atrophy (Hardy 1967, Hummel et al 1966, Pearse 2006b, Wijnands et al 1966). Involution is characterized by a reduction in size of the thymus, a loss of cortical lymphocytes, and a loss of corticomedullary demarcation (Pearse 2006b), but the thymus does not completely involute (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2007, Hardy 1967, Percy & Barthold 2007, Ward et al 1999).
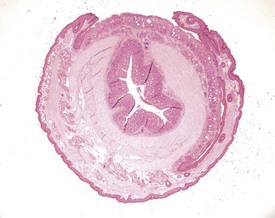
Hassall’s corpuscles are very small or absent in the mouse thymus (Pearse 2006a, Percy & Barthold 2007, van Rees et al 1996, Ward et al 1999). Thymic cysts develop from embryonic remnants arising from the thymopharyngeal duct, or dilatation of thymic tubular structures, and they increase in number with age (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2007, Pearse 2006a, b, Ward et al 1999, Wijnands et al 1966). The cysts are lined by ciliated columnar epithelium and usually contain amorphous, eosinophilic material (Fig. 4.13).
Embryonic thymic remnants can give rise to ectopic thymic tissues in the thyroids or parathyroids, and occasionally ectopic parathyroid tissue is seen in the thymus (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985, 2007, Pearse 2006a, Percy & Barthold 2007, Ward et al 1999). Lymphoid hyperplasia of the thymus occurs in mice over six months of age, is more common in females, and may be seen in association with thymic involution (Faccini et al 1990, Frith et al 1985, 2001, Pearse 2006b, Ward et al 1999) (Fig. 4.14). Lymphofollicular structures with germinal centres can be seen at the corticomedullary junction in aged mice, in association with atrophic cortical changes and hyperplastic medullary changes (Berdanier 2004, Dumont & Robert 1980, Pearse 2006b) (Fig. 4.15).
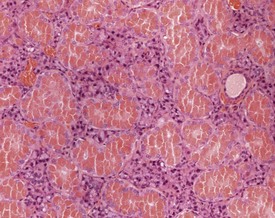
Normal bone marrow is composed of hematopoietic elements including granulocytic, erythrocytic, megakaryocytic elements and stem cells. Each of these may undergo hyperplasia in response to a physiological need. The most common form of hyperplasia in murine bone marrow is an increase in the granulocytic elements in response to inflammatory lesions elsewhere in the animal (Faccini et al 1990, Frith et al 1985, 2001) (Fig. 4.16).
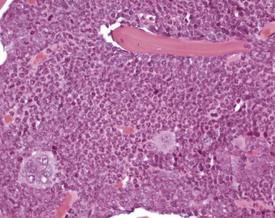
FIGURE 4.16 Increased granulopoiesis in the bone marrow of the sternum of an 18-week-old, male, CD-1 mouse. ×400.
Atrophy of the bone marrow is rare in mice, but may occur occasionally, and is characterized by a decrease in number of all elements of the marrow, and increased adipose tissue replacement (Faccini et al 1990, Frith & Ward 1988, Frith et al 1985).
Respiratory system
Various reviews of the embryology, anatomy, structure and physiology of the lungs and the upper respiratory tract are available (Braun et al 2004, Dixon et al 1999, Harkema et al 2006, Herbert & Leininger 1999, Kuhn 1985, Pack et al 1981, Pinkerton et al 1996, Renne et al 1992, Reznik 1990).
The variations in the structure and distribution of different epithelia within the upper respiratory tract require consistent sectioning of the nasal turbinates and larynx. This is important to ensure consistency in evaluation of the different structures, which can have different susceptibilities to pathological changes, particularly as a result of inhalation exposure to test materials (Herbert & Leininger 1999, Kittel et al 2004, Renne et al 1992, Ruehl-Fehlert et al 2003). In order to aid in the identification of the different epithelia within the nasal turbinates, diagrams of coronal and sagittal sections of the nasal passages of the rat and mouse have been produced (Mery et al 1994).
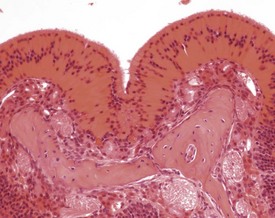
One of the most commonly encountered changes in the nasal turbinates of the mouse is the presence of intracytoplasmic, hyaline inclusions (eosinophilic globules, eosinophilic secretory inclusions, hyaline droplet accumulation) (Berdanier 2004, Braun et al 2004, Dungworth et al 2001, Herbert & Leininger 1999, Leininger et al 1996, Monticello et al 1990, Percy & Barthold 2007, Renne et al 2009, Ward et al 2001). These inclusions can occur at multiple sites within the turbinates and can affect all types of epithelium. The brightly eosinophilic material is observed within the cytoplasm of the mucosal epithelial cells and of submucosal glands and ducts and probably represents proteinaceous secretory material accumulating in the cells (Fig. 4.17). Eosinophilic crystals can also occur both within the epithelium and extracellularly. These changes occur in many strains of mice, and increase in severity with age.
Another common change in the nasal turbinates of mice, which increases in severity with age, is the accumulation of hyaline material in the nasal ventral septum (Leininger et al 1996, Monticello et al 1990, Percy & Barthold). This material has been identified as amyloid (Herbert & Leininger 1999, Percy & Barthold 2007), but the lack of positive staining with Congo Red and positive staining for collagen suggests to some authors that the material is not amyloid, but a combination of collagen and an amorphous material produced by nasal gland epithelial cells (Brayton 2007, Doi et al 2007, 2009, 2010) (Fig. 4.18). Doi et al (2010) have reported sex differences in the occurrence of eosinophilic substance in the nasal turbinates of B6C3F1 mice, with males showing a greater degree of deposition than females. Inflammatory changes in the turbinates occasionally occur in association with foreign bodies, infection or injury (Herbert & Leininger 1999) and may be associated with dysplastic dental changes encroaching into the nasal passages (Leininger et al 1996).
Spontaneous, age-related changes in the larynx are uncommon, but changes can occur commonly from inhalation of irritant materials (Herbert & Leininger 1999).
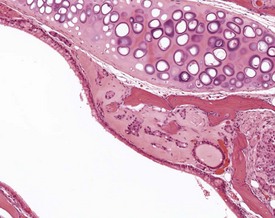
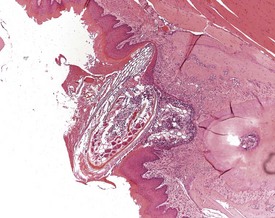
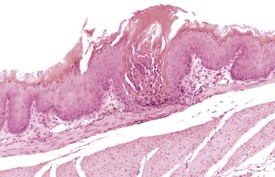
Foreign-body reactions are occasionally observed in the laryngeal ventral pouch. Foreign material is seen in the lumen of the ventral pouch, associated with an inflammatory reaction and hyperplasia of the laryngeal epithelium. These changes are not limited to studies utilizing the inhalation route of exposure, but can be seen in all types of study (Fig. 4.19). One lesion described as the only common change associated with aging in the larynx of B6C3F1 mice in NTP lifetime studies, is the dilatation of laryngeal glands with the accumulation of eosinophilic crystalline material (Leininger et al 1996).
Spontaneous, age-related changes in the trachea are uncommon, but changes can occur from inhalation of irritant materials (Herbert & Leininger 1999). Intracytoplasmic hyaline inclusions can be seen in the epithelium of the trachea, bronchi and bronchioles, with a similar appearance to those seen in other regions of the respiratory tract (Dungworth et al 2001, Ward et al 2001, Yang & Campbell 1964). The incidence and severity of this change increases with age. Eosinophilic crystals can also be associated with this lesion, and can accumulate in the lumen of tracheal glands (Fig. 4.20).
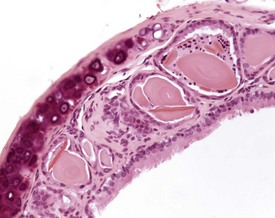
FIGURE 4.20 Trachea: dilated tracheal glands containing eosinophilic amorphous material and eosinophilic crystals. ×200.
Pulmonary hair emboli are occasionally seen in the lungs of mice following intravenous injection into the caudal veins. Hair fragments in the circulation are trapped in the lung vasculature, phagocytosed, and can be seen surrounded by foreign-body giant cells (Ernst et al 1996, Faccini et al 1990, Innes et al 1958, Kast 1985).
Osseous metaplasia is occasionally observed in the lungs of mice, although at a lower incidence than seen in rats. These small foci are usually composed of osteoid or bone and may show early mineralization or calcification. There is usually no reaction in the surrounding parenchyma (Braun et al 2004, Dixon et al 1999, Ernst et al 1996, Faccini et al 1990) (Fig. 4.21).
Arterial plaque is a lung-specific change occasionally seen in the lungs of mice. Developing from the tunica media of medium to large pulmonary arteries, there is a focal protrusion into the lumen of the vessel. The lesion does not occlude the lumen and is covered by endothelial cells. There is no associated inflammatory reaction, and in older lesions mineralization develops within the plaque (Ernst et al 1996, Rehm et al 1985b) (Fig. 4.22). The walls of the major pulmonary veins of the lungs in mice contain cardiac muscle (Best & Heath 1961, Dixon et al 1999, Krinke 2004, Percy & Barthold 2007) (Fig. 4.23).
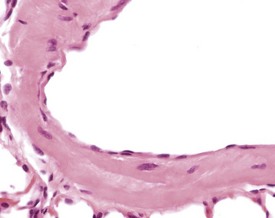
FIGURE 4.23 Lungs with striated (cardiac) muscle in wall of pulmonary vein of a two-year-old, female, CD-1 mouse. ×400.
Eosinophilic crystal accumulation in alveolar spaces with associated inflammatory-cell infiltration is seen occasionally in routine toxicity studies (Dixon et al 1999, Ernst et al 1996, Frith et al 2007), and accumulations of eosinophilic material within alveolar macrophages is associated commonly with bronchioloalveolar tumors in carcinogenicity studies (Dungworth et al 2001, Frith & Ward 1988, Green 1942). The eosinophilic material has been considered to originate from breakdown products of granulocytes or hemoglobin (Dixon et al 1999, Ernst et al 1996, Marshall et al 1988, Murray & Luz 1990) (Fig. 4.24).
Eosinophilic crystalline pneumonia is an idiopathic disease affecting certain strains and stocks of mice; it is characterized by accumulations of fine needle-like eosinophilic crystals in macrophages and multinucleate giant cells within alveolar and bronchiolar spaces. These are accompanied by mixed inflammatory cell infiltrates. The disease can range from a mild, subclinical condition to a severe and fulminating change resulting in respiratory distress and death (Braun et al 2004, Hoenerhoff et al 2006, Ward et al 2001).The crystalline material has been identified as being composed primarily of Ym1 protein, a chitinase-like protein associated with neutrophil granule products and secreted by activated macrophages (Braun et al 2004, Guo et al 2000, Hoenerhoff et al 2006, Renne et al 2009). This disease occurs with a high incidence in certain strains of mice, such as the C57BL/6 and 129Sv strains (Guo et al 2000, Hoenerhoff et al 2006, Ward et al 2001).
Focal accumulations of macrophages (alveolar histiocytosis) are a common incidental finding in the lungs (Braun et al 2004, Dixon et al 1999, Faccini et al 1990, Frith & Ward 1988, Frith et al 2007, Renne et al 2009), and can be seen commonly in subpleural areas of aging mice lungs.
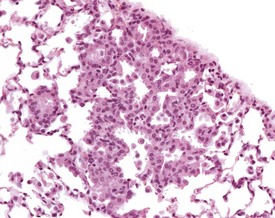
Focal hyperplasia of the alveolar epithelium is occasionally seen in older mice, particularly those with a high background incidence of bronchioloalveolar tumors, and the incidence increases with age (Braun et al 2004, Dixon et al 1999, Dungworth et al 2001, Faccini et al 1990, Frith et al 2007). Accumulations of alveolar macrophages may be associated with the hyperplastic cells. The affected alveoli are lined by uniform, hypertrophic epithelium. Cytoplasmic basophilia and enlarged nuclei are apparent. The cells form a single layer that is contiguous throughout the area of hyperplasia and the margins of the lesion are indistinct (Renne et al 2009) (Fig. 4.25).
Gastrointestinal system
Reviews of the embryology, anatomy, histology and physiology of the oral cavity, gastrointestinal tract, teeth and salivary glands are available (Berdanier 2004, Botts et al 1999, Leininger et al 1999, Long & Leininger 1999a, Shackelford & Elwell 1999, Tucker 2007).
With the exception of abnormalities of the teeth, there are usually very few spontaneous lesions of the oral cavity of the mouse (Leininger et al 1999). Periodontal disease associated with the impaction of hair, food or nesting material in the gum adjacent to molars and incisors, occurs occasionally, resulting in inflammatory infiltration of the periodontal tissues (Fig. 4.26).
The impacted areas can develop into ulceration, abscessation and the formation of periodontal cysts, with disruption of the normal growth of the associated dental tissue and bone (Long & Leininger 1999a, Losco 1995, Percy & Barthold 2007, Sakura 1997). Periodontal abscesses can develop involving the adjacent tissues of the head and may infiltrate the nasal turbinates (Leininger et al 1996, Percy & Barthold 2007).
The incisors of rodents grow continuously throughout their life, and therefore damage to the teeth can predispose the teeth to abnormal development and malformations, either as a result of damage by repeated clipping of teeth, traumatic damage, or as a result of inflammation (Long & Leininger 1999a, Losco 1995, Sakura 1997). This can result in dental dysplasia with disruption of the normal growth patterns of odontoblasts and ameloblasts, and the abnormal deposition of mineralized dental material (Berdanier 2004, Leininger et al 1996, Long & Leininger 1999a, Losco 1995, Maekawa et al 1996a, Sakura 1997, Weber 2007).
Teeth are not routinely presented for histopathological examination in pre-clinical studies, and are most commonly examined in association with sections of the nasal turbinates. As a result, the reported incidence of more subtle changes in the teeth of mice may be artificially lower than would occur if sections of teeth were routinely examined. Long & Herbert (2002) presented information on the occurrence of calcified bodies (denticles) within the dental pulp, which they considered could affect the normal growth pattern of the teeth and lead to malformations and malocclusion of mouse incisors.
Three paired salivary glands, the submandibular (submaxillary), parotid and sublingual glands, are closely associated and located in the subcutaneous tissue of the ventral neck. Salivary glands are also located at the base of the tongue and may be presented in longitudinal sections of the tongue (Ruehl-Fehlert et al 2003).
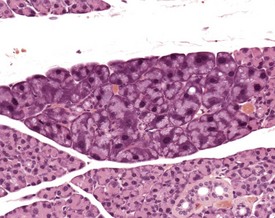
There is a striking sexual dimorphism in the structure of the submandibular salivary glands which becomes apparent once males reach sexual maturity (Fig. 4.27a & b). The gland of the male is larger, and the granular convoluted ducts are larger and contain much more prominent eosinophilic granules (Berdanier 2004, Botts et al 1999, Brayton 2007, Faccini et al 1990, Frith et al 2007, Frith & Townsend 1985, Frith & Ward 1988, Hummel et al 1996, Krinke 2004, Maekawa et al 1996a, Ogawa 2003, Percy & Barthold 2007, Seely 1996b, Tucker 2007). This appears to be under the control of testosterone, as the difference in morphology is not apparent in young males, and castration of male mice results in a reduced development of granular convoluted ducts (Smith & Frommer 1975).
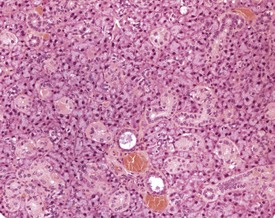
FIGURE 4.27b Eighteen-month-old, female, CD-1 mouse with less prominent eosinophilic granules. ×200.
Lymphocytic infiltration of the salivary glands is a common finding, and increases in incidence with age (Berdanier 2004, Botts et al 1999, Faccini et al 1990, Maekawa et al 1996a, Seely 1996b). Atrophy occurs occasionally in the submandibular and parotid salivary glands; however, it is uncommon in the sublingual glands (Botts et al 1999, Frith et al 2007, Frith & Ward 1988, Seely 1996b). Atrophy usually affects a single lobule with reduction in size of acini and a reduction in the diameter and eosinophilic granule content of the granular convoluted ducts. An apparent increase in the number of ductular elements may be as a result of the reduced size of the acinar component and an apparent crowding of the remaining ductular elements (Fig. 4.28).
Basophilic hypertrophic foci are seen occasionally in the parotid salivary glands (Berdanier 2004, Botts et al 1999, Chiu & Chen 1986, Frith et al 2007, Krinke 2004, Seely 1996b). These can occur singly or in multiple locations. There are sharply demarcated foci of enlarged cells with increased basophilia. There appears to be no associated capsule formation, compression of adjacent tissue or inflammatory involvement. The etiology of this change is unclear (Fig. 4.29).
Spontaneous lesions of the tongue are relatively rare in mice. Certain strains of inbred mice develop spontaneous calcification of the muscle layers of the tongue at a very young age (Imaoka et al 1986, Maekawa et al 1996a, Yamate et al 1987), but this is not apparent in CD-1 mice. Hemorrhage and degeneration of the muscle can be seen occasionally, resulting from blood sampling via the sublingual vein.
Few spontaneous lesions occur in the esophagus. Gavage accidents may occur rarely, resulting in rupture of the esophagus, with a consequent associated inflammatory reaction of the muscularis and serosa (Frith & Ward 1988, Leininger et al 1999) if the animal survives. Hyperkeratosis has been described as the most common lesion in the esophagus of aging B6C3F1 mice (Leininger et al 1999, Maekawa et al 1996a), but occurs rarely in CD-1 mice. Mega-oesophagus is occasionally noted in older mice.
Minor inflammatory lesions of the forestomach are occasionally seen, often associated with focal erosions or ulcerations, and squamous hyperplasia and hyperkeratosis (Betton et al 2001, Faccini et al 1990, Frith et al 2007, Leininger et al 1999, Maekawa et al 1996a) (Fig. 4.30).
Hyperplastic lesions of the forestomach are usually focal in nature, but diffuse hyperplasia and hyperkeratosis is associated with reduced food intake in mice (Faccini et al 1990, Leininger et al 1999). The pathologist must be careful to distinguish hyperplasia from the thickening seen at the limiting ridge at the junction of the non-glandular and glandular stomach.
Developmental anomalies are rare, but ectopic tissues have been reported in the stomach, such as hepatocytes in the submucosa/lamina propria near the limiting ridge, and ectopic pancreatic tissue in the submucosa (Leininger et al 1990, 1999, Maekawa et al 1996a). Inflammatory lesions occur less frequently in the glandular stomach.
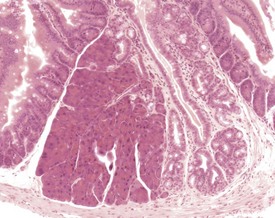
Adenomatous hyperplasia of the glandular stomach occurs commonly in aging CD-1 mice, with a higher incidence in females compared to males. This change has been variously described as gastric hyperplasia, glandular hyperplasia, hypertrophic gastritis, proliferative gastritis, dysplasia of gastric epithelium and fundic mucosal hyperplasia (Betton et al 2001, Faccini et al 1990, Frith & Ward 1988, Greaves & Boiziau, 1984, Leininger et al 1999, Maekawa et al 1996a, Rehm et al 1987). The incidence and severity of this change is strain- and age-dependent, and is reported to occur more frequently in densely housed mice than in singly housed mice (Greaves & Boiziau, 1984). There is no clinical manifestation of this change, and it is not associated with parasitic infection, although an association with hormonal disturbances and kidney disease has been suggested (Rehm et al 1987).
The early features of adenomatous hyperplasia of the glandular stomach are focal hyperplasia of the gastric mucosa with increased epithelial basophilia, elongation of the crypts and occasional cystic glands. As the lesion progresses the mucosa becomes increasingly thicker with a diffuse epithelial hyperplasia, disorganization of the normal glandular structure, prominent branching of elongated crypts and cyst formation, and an accompanying inflammatory infiltration in the associated submucosa. In severe cases there is extension of disorganized mucosa through the muscularis to the serosa. This is not, however, considered to be a sign of malignancy. Within the cystic glands, hypertrophic cells containing eosinophilic inclusions can be seen (Figs 4.31–4.33). The cause of the eosinophilic intracytoplasmic inclusions in the gastric mucosa is unknown.
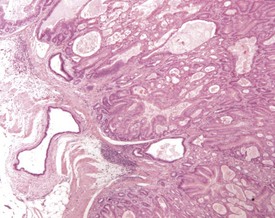
FIGURE 4.32 Higher-power view of Fig. 4.31 showing cystic dilatation of the mucosal glands and extension of the disorganized mucosa through the muscularis. ×40.
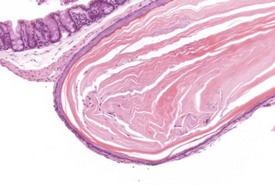
Congenital lesions in the intestine of the mouse are relatively rare, although ectopic pancreatic tissue can occasionally be seen in the submucosa of the duodenum (Shackelford & Elwell 1999) (Fig. 4.34). Epidermal inclusion cysts occur occasionally in the large intestine (Frith et al 2007). These cysts occur in the muscularis of the colon and rectum, are lined by squamous epithelium, and contain desquamated keratin (Fig. 4.35).
Diverticuli occur occasionally in the large intestine (Frith et al 2007). These should be examined carefully to ensure that they are not a result of artifactual folding of the section. The non-proliferative nature of the overlying mucosa should allow the differentiation between diverticuli and adenocarcinoma (Fig. 4.36).
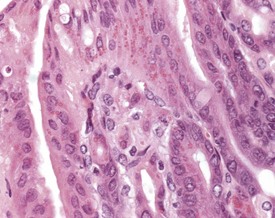
The Paneth cells of the small intestine of the mouse contain prominent zymogen granules (Krinke 2004, Percy & Barthold 2007, Shackelford & Elwell 1999) (Fig. 4.37). Spontaneous degenerative and inflammatory lesions are relatively uncommon in the mouse intestine, and occur more frequently in the stomach than in the intestine (Frith et al 2007, Maekawa et al, 1996b, Shackelford & Elwell 1999).
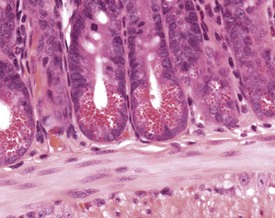
FIGURE 4.37 Prominent zymogen granules in the Paneth cells of the ileum of a 20-week-old, male, CD-1 mouse. ×400.
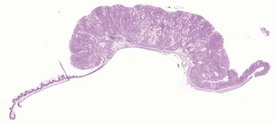
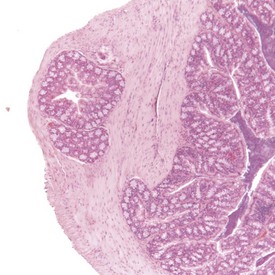
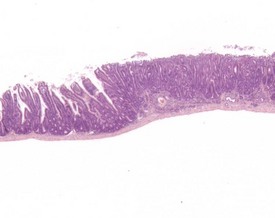
Spontaneous hyperplastic lesions are also rare in the small and large intestine, but avillous hyperplasia of the duodenum is seen in CD-1 mice (Faccini et al 1990). This change is characterized by thickening of the duodenal mucosa without formation of villi, which occur at the first part of the duodenum adjacent to the pyloric sphincter (Betton et al 2001, Faccini et al 1990, Rowlatt et al 1969). These changes are sometimes referred to as epithelial plaques. Hyperplasia usually involves all cells of the epithelium, including the underlying Brunner’s glands. In more severe cases, dilated mucosal glands can extend towards the submucosa and be interspersed between dilated Brunner’s glands. The overlying epithelium lacks villous projections and can often show erosions or ulcerations with an associated inflammatory infiltration. Submucosal inflammation and edema are also present (Figs 4.38, 4.39). These changes are considered to be reactive in nature because of the inflammatory involvement and the hyperplasia of more than one type of cell (Betton et al 2001, Rowlatt et al 1969).
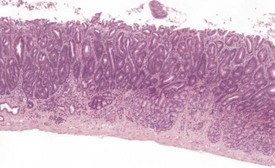
FIGURE 4.39 Higher power view of Fig. 4.38 showing hyperplastic epithelium with erosion of surface and inflammatory cell infiltration. ×40.
Intussusception is occasionally seen in the large and small intestine, and rectal prolapse can occasionally occur. These changes are often associated with intestinal neoplasms, parasites or other conditions associated with irritation of the intestine (Betton et al 2001, Frith & Ward 1988, Frith et al 2007, Mahesh Kumar et al 2004, Rowlatt et al 1969, Shackelford & Elwell, 1999). These conditions can lead to intestinal obstruction, inflammation, necrosis and death. Rectal prolapse is characterized by eversion of the mucosal surface of the rectum through the anus (Fig. 4.40).
Pinworms are occasionally seen in the lumen of the colon (Fig. 4.41). Cross sections of the worms, usually Syphacia obvelata or Aspiculara tetraptera, are seen in the lumen. Inflammatory reaction in the adjacent epithelium is not normally associated with the presence of the parasites (Faccini et al 1990, Frith et al 2007). Syphacia obvelata infection is not associated with goblet cell hyperplasia in the colon of mice (Marillier et al 2008).
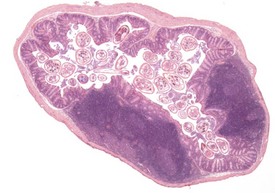
FIGURE 4.41 Adult pinworms (Syphacia obvelata) in the lumen of the colon of an 18-month-old, male, CD-1 mouse. ×20.
Focal adipose tissue necrosis in the mesentery is a relatively common finding in mice and rats. These lesions are grossly visible as yellow nodules. Histologically, areas of adipose tissue necrosis are circumscribed by a granulomatous inflammatory reaction, with fibrous tissue and areas of hemorrhage, acute inflammatory infiltration and pigment deposition. In chronic lesions, areas of mineralization may be present. The etiology of these lesions may be related to focal ischemia (Maekawa et al 1996b, Shackelford & Elwell 1999).
Liver and biliary system
The embryology, anatomy, histology and physiology of the liver and gall bladder are reviewed by Harada et al (1999) and Thoolen et al (2010), and the functional aspects of liver structure are reviewed by Malarkey et al (2005).
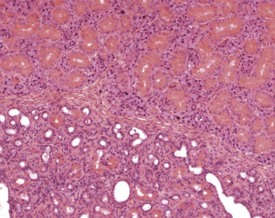
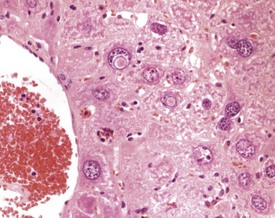
Congenital lesions in the liver of mice are relatively unusual. Hepatodiaphragmatic nodules, formed as a result of protrusion of the median liver lobe through the diaphragm, occur at a much lower incidence than in rats. Ectopic tissues in the liver are also rare, but ectopic renal tissue can occasionally be seen in the mouse liver (Harada et al 1999) (Fig. 4.42).
Extramedullary hemopoiesis (EMH) is a normal feature of the fetal mouse liver, but this function is lost as the bone marrow takes over this function in the adult mouse. Extramedullary hemopoiesis can occur in the adult mouse liver as a response to functional needs, however, for example in response to anemia, infectious disease or neoplasia (Faccini et al 1990, Frith & Ward 1979, 1988, Frith et al 2007, Harada et al 1996, 1999, Jones 1967, Thoolen et al 2010). The nature of the cellular components of EHM can vary depending on the nature of the initiating factors, but foci may be found in sinusoids, around central veins or in periportal areas (Fig. 4.43).
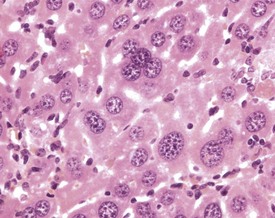
Some of the more striking features of the aging mouse liver are the presence of cytomegaly, karyomegaly, intranuclear and intracytoplasmic inclusions (Berdanier 2004, Faccini et al 1990, Frith & Ward 1979, 1988, Frith et al 2007, Harada et al 1996, 1999, Jones 1967, Percy & Barthold 2007, Thoolen et al 2010, Toth & Sugar 1985, Tucker & Baker 1967, van Zweiten & Hollander 1985). These changes increase in incidence and severity with age, and commonly occur within the normal aging liver. Some of these changes are also evident in neoplastic hepatocytes.
The relative number of binucleate and multinucleate hepatocytes in the liver increases with age, either as a result of the failure of dividing cells to separate during mitosis, or by the fusion of cells (Wilson & Leduc 1948). The presence of very large, hepatocellular nuclei in enlarged hepatocytes also increases with age, and represents increased chromosome numbers, as a result of failure of division of nuclei during mitosis. Polyploid nuclei may contain two, four or eight times the normal amount of nuclear DNA (Harada et al 1999, Jones 1967) (Fig. 4.44).
Intranuclear and intracytoplasmic inclusions are frequently observed in the aging mouse liver. Intranuclear, eosinophilic inclusions occur commonly in the aging mouse liver (Fig. 4.45). Eosinophilic, homogeneous material forms distinct spherical inclusions in the nucleus, and these are considered to be invaginations of cytoplasmic material (Frith & Ward 1988, Frith et al 2007, Harada et al 1999, Jones 1967, Thoolen et al 2010, Toth & Sugar 1985).
Eosinophilic inclusions are seen occasionally in the cytoplasm of hepatocytes, the significance of which is unclear, but they probably represent a disturbance of protein production by the rough endoplasmic reticulum (Frith & Ward 1988, Jones 1967, Toth & Sugar 1985). They are observed frequently in benign hepatocellular tumors, but they may also occur in normal liver cells (Fig. 4.46).
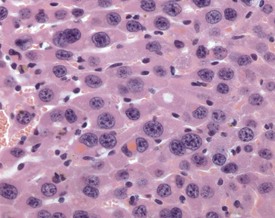
FIGURE 4.46 Intracytoplasmic, eosinophilic inclusions in the hepatocytes of an 18-month-old, male, CD-1 mouse. ×400.
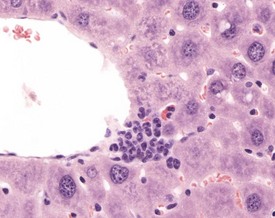
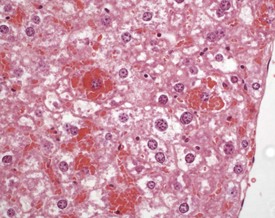
Intracytoplasmic accumulation of erythrocytes is also occasionally observed as a spontaneous change in the aging mouse liver (Harada et al 1999, Thoolen et al 2010, Tucker & Baker 1967). The cytoplasm of the enlarged hepatocyte is seen to contain intact erythrocytes. This is usually a focal change and is considered to represent erythrophagocytosis by the hepatocyte (Fig. 4.47).
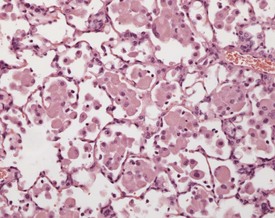
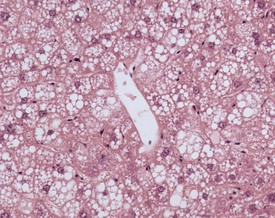
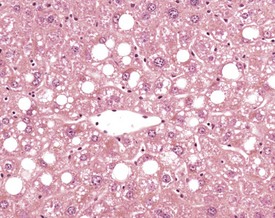
A variety of degenerative lesions are seen in the liver of aging mice. Hepatocellular vacuolation is a common incidental finding in the liver of aging mice, and can occur as a focal, zonal or diffuse change. Vacuolation is more commonly visualized in males. The vacuoles are the result of lipid accumulation in the cytoplasm of the hepatocyte, and appear as clear, distinct, round vacuoles in the cytoplasm on H&E-stained sections of liver (Faccini et al 1990, Frith & Ward 1988, Frith et al, 2007, Harada et al 1996, 1999, Percy & Barthold 2007, Thoolen et al 2010, Tucker & Baker 1967). Macrovesicular vacuolation is characterized by the presence of a single large vacuole in the cytoplasm, with displacement of the nucleus. Microvesicular vacuolation is characterized by the accumulation of numerous small, round vacuoles in the cytoplasm, with no displacement of the nucleus. The lipid in the vacuoles is lost during routine processing of formalin-fixed tissues, but O Red O staining of frozen sections can confirm the presence of lipid (Figs 4.48, 4.49).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree