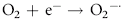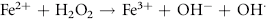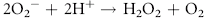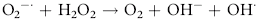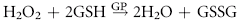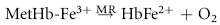Chapter 88 Methemoglobinemia
PATHOPHYSIOLOGY
Oxidation in the Erythrocyte
Erythrocytes are especially vulnerable to oxidative damage because they carry oxygen, are exposed to various chemicals in plasma, and have no nucleus or mitochondria.1,3 The lack of cellular organelles renders the membrane the deformability necessary to navigate capillary beds, but results in a cell that is incapable of producing proteins or performing efficient energy production.1 They therefore have a finite number of cell proteins and are reliant on anaerobic respiration to generate energy and reducing agents.1 Oxidants continuously generated in vivo include hydrogen peroxide (H2O2), superoxide free radicals (O2−), and hydroxyl radicals (OH·) (Box 88-1).1,3,4 Hemoglobin can undergo autooxidation as an electron is pulled off the hemoglobin onto an oxygen molecule, resulting in the generation of metHb and O2−.1,3 Free radicals may also extract electrons by oxidizing deoxyhemoglobin.3 In contrast oxidant toxins can donate an electron to oxyhemoglobin, creating metHb and hydrogen peroxide (Box 88-1).3
Box 88-1 Chemical Reactions Resulting in Free Radical Formation, Their Removal, and Methemoglobin Reduction
Modified from Engelking LR: Textbook of veterinary physiological chemistry, Jackson Hole, WY, 2004, Teton NewMedia.
Despite their limited capacity to produce energy and proteins, erythrocytes have many mechanisms to protect themselves from oxidative damage. These include superoxide dismutase, catalase, glutathione peroxidase, glutathione, and metHb reductase (cytochrome b5 reductase) (see Box 88-1).1,3 Glutathione is a tripeptide produced in erythrocytes and composed of glutamic acid, cysteine, and glycine and contains an easily oxidizable sulfhydryl (SH) group.3 It is a powerful antioxidant that operates as a free radical scavenger. Reducing agents such as nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NADH) are instrumental in reducing oxidized glutathione and metHb back to functional molecules (see Box 88-1).1,3,4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree