Chapter 14 Metabolic Diseases
The common metabolic problems of early lactation, milk fever and ketosis, are really management diseases. At the herd level, disease does or does not occur as a function of how cows are fed and handled during the late dry period and during transition to the nutrient-dense rations needed to support high milk production in early lactation. Because infectious diseases are more effectively controlled by sound immunization, the economic importance of these common metabolic disorders and their prevention by sound nutritional and herd management has assumed ever greater relevance on the modern dairy. Feeding management includes sources, storage, preparation, ration formulation, delivery, and access. Good feeding management must be coupled with providing an environment as comfortable as possible to facilitate maximal feed consumption. In investigating herd problems of excessive metabolic diseases, all these factors must be considered. Individual cows may be predisposed to metabolic problems as a result of improper body conditioning, concurrent illness, genetics, and any other events that may decrease dry matter intake. In addition to calcium, the other macrominerals of relevance in dairy cattle are potassium, magnesium, and phosphorous, and although disorders involving these elements are of far lesser importance than hypocalcemia, they will also be considered within this chapter.
KETOSIS: CAUSES, CLASSIFICATION, AND PATHOPHYSIOLOGY
Hepatic lipidosis may take at least three forms: (1) clinically silent in subclinical ketosis, (2) chronic fat mobilization following early-onset periparturient ketosis with an individual susceptibility as a result of either genetics and/or periparturient overconditioning, and (3) periparturient ketosis in the obese cow with massive lipid accumulation in the liver within the first days of lactation.
Clinical Signs and Diagnosis of Ketosis
Primary or spontaneous ketosis is most common in the first month of lactation, with the majority of cases occurring between 2 and 4 weeks of lactation. Cows with either ketosis early (first week) in lactation or cows with persistent ketosis beyond 4 weeks of lactation are most likely to have more marked hepatic lipidosis. Cows with primary ketosis have reduced feed intake of total mixed rations (TMRs) and may prefer forages over concentrates if ingredient fed. Temperature, pulse, and respiration are normal or occasionally subnormal. The rumen in TMR-fed cows will be reduced in volume, have a lower contraction frequency, and also typically have a small fiber mat. In ingredient-fed cows, the rumen may be normal in size but with a large, doughy fiber mat. It is common to hear the heartbeat while listening to the rumen of affected cows. Ketones may be detected in the breath, urine, or milk. Some sensitive individuals can easily recognize this odor. A urine test for acetoacetate is widely available and is the most sensitive test, although specificity is not as high as with milk ketone tests. A color change to purple indicates the presence of acetoacetate (Figure 14-1). The rate and intensity of change are indicative of acetoacetate concentration, but the urine acetoacetate test may be affected by the hydration status of the cow and the concentration of the urine. Many cows with primary ketosis give a strong purple color on the urine test, although the urine of individuals with hepatic lipidosis may only cause a lighter purple coloration. The manure is drier in consistency than herdmates at the same stage of lactation. Affected cows appear dull with a dry hair coat and piloerection. Neurological signs such as persistent licking at herself or objects, aggressive behavior, and unusual head carriage may be seen with nervous ketosis. The pathogenesis of nervous ketosis is unknown. Inability to rise or ataxia resulting from weakness may be seen in some cows with primary ketosis, and these signs are directly related to hypoglycemia. Metabolic acidosis may occur in some cows and, although unpredictable, can be severe (bicarbonate of as low as 12 mEq/L) in a few cows.
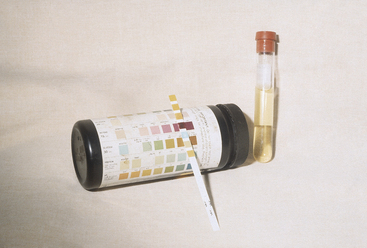
Figure 14-1 Urine ketostrip with urine-positive reaction to acetoacetate from a cow with primary ketosis.
Cows with persistent ketosis for 1 to 7 weeks usually have hepatic lipidosis. Ultrasound examination or biopsy of the liver (Figure 14-2) can be used to confirm hepatic lipidosis, but this is seldom required because the diagnosis is easy but treatment more difficult. Cows with chronic ketosis/fat mobilization and hepatic lipidosis lose considerable amounts of weight, have a poor appetite, but continue to produce moderate amounts of milk considering their poor feed intake (Figure 14-3). Affected cows may appear weak, which could be caused by hypoglycemia, muscle weakness from fatty accumulation in muscle, and/or hypokalemia. Some cows may die, be sold, or have complications caused by frequent treatment (e.g., phlebitis from glucose administration, oral trauma from forced feeding). Serum concentrations of hepatic-derived enzymes (aspartate aminotransferase [AST], gamma glutamyl transferase [GGT], and sorbitol dehydrogenase [SDH]) are often elevated, and serum cholesterol is frequently low in cows with hepatic lipidosis. However, these values are not consistently abnormal. Serum cholesterol generally returns toward normal value as the cow begins to eat better.
Cows that are overconditioned before parturition and have periparturient ketosis (although a urine ketone test may be only weakly positive) rapidly develop hepatic lipidosis and have life-threatening illness (Figure 14-4). These cows have recurrent hypocalcemia and recumbency and, because of their heavy weight, often develop fatal myopathy (Figure 14-5). Most of these obese/periparturient ketosis/hepatic lipidosis cows have retained placenta and may die of septic metritis even without a fetid smelling discharge (Figure 14-6). Their predisposition to sepsis with mild to moderate metritis may be caused by excessive fat deposition in the liver and diminished hepatic macrophage (Kupffer cells) function. Affected cows may also develop septic mastitis with repeated episodes of recumbency.
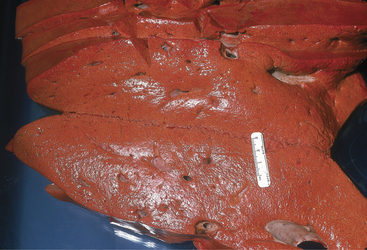
Figure 14-4 Severe hepatic lipidosis observed at necropsy. The liver was from a recently fresh and obese cow.
Treatment
Treatment for ketosis is aimed at restoring energy metabolism to normal for milk production. The three most commonly used treatments are 500 ml of 50% dextrose given intravenously (IV) once or twice, glucocorticoid administration (e.g., 10 to 20 mg of dexamethasone once), and 300 ml of propylene glycol orally once or twice a day for 5 days. These treatments may be combined to suit the needs of the case and the abilities of the herdsman. The propylene glycol should be given as a drench and not mixed in the feed. Full recovery requires the return to normal feed intake, and supportive therapy may need to be continued for several days to allow time for the cow to maintain normoglycemia. Offering a choice of feedstuffs (i.e., brewer’s yeast) may help in restoring the cow’s appetite. Cows with nervous ketosis can be treated with chloral hydrate (40 g orally daily), which serves as both a sedative and as a substrate for glucogenic-producing bacteria.
Treatment of periparturient overweight cows with ketosis and hepatic lipidosis is intensive. Affected cows are administered IV fluids to combat hypotension and lactic acidosis. Glucose and calcium are often added to the fluids, although baseline blood glucose levels may be high in some of the cows. The cows should be force fed as described above and have only limited milk removed (if there is mastitis in a quarter, it should be stripped and intramammary antibiotics administered). Insulin therapy can be used as described previously for cows with chronic fat mobilization. Reduced neutrophil and hepatic macrophage function in these cows may allow septic conditions such as even mild metritis or mastitis to overwhelm the patient. Fresh feed, clean water, and salt should be available, and the cow should be housed in either a large well-bedded box stall with excellent footing or in a grass paddock. Along with sepsis, musculoskeletal injury is the most common reason for euthanasia of overweight cows with periparturient hepatic lipidosis. Every effort should be made to maintain calcium levels within normal limits by either slow continuous infusion or SQ administration; ideally ionized calcium should be closely monitored and the cow housed in an area that will provide the best comfort for standing up and lying down. If there has been any difficulty in rising, the cow should be administered flunixin meglumine (500 mg once or even twice daily if needed). The knees should be wrapped with soft cotton bandages to provide protection to the carpus area, which is often the first anatomical site to be adversely affected in cows that have difficulty rising. Although lipotropic medications such as choline and methionine are used by some clinicians for cattle with hepatic lipidosis, their value in the treatment probably is not significant. If lipotropic medications are used, rumen-protected choline is preferred. Electrolyte imbalances also should be addressed should laboratory facilities exist that allows easy assessment of these values.
Ketosis as a Herd Problem
Because all cows undergo physiological accumulation of lipid in the liver during the periparturient period, conditions that lead to excessive lipid mobilization are most likely to result in severe hepatic lipidosis and ketosis. Obesity or other diseases that restrict feed intake are both potential causes. Conversely, the force feeding via rumen fistula of the difference between intake at 3 weeks prepartum and voluntary intake until calving reduced the increase in liver triglyceride accumulation from 23% to 16%. Most data suggest that an attempt should be made to gradually increase nonfiber carbohydrates (NFCs) in the last 2 weeks of gestation in an attempt to increase dry matter intake and maintain a positive energy balance in the cow. An additional benefit is an increase in plasma insulin, which inhibits lipolysis. This increase in NFC (to between 34% and 36%) should be a gradual increase such that the cow will be continually increasing caloric intake into the first few days of lactation. Further restriction of intake in the late dry period when a decline normally occurs can be a herd problem. A separate feeding group has been recommended for the springing cows with a diet formulated to greater nutrient density than for early dry cows. Mismanagement of this group has occurred, leading to outbreaks of postpartum ketosis. Dr. Guard describes a herd with a ketosis problem that offered its close up cows an appropriate ration. There were 3 in of bunk space for 15 to 25 cows. The area in front of the bunk was a deep mudhole. In addition, there was an electric fence surrounding the bunk and strung across the top to prevent cows from stepping into the feed. Creating a new 20-m feed bunk away from mud and electricity appeared to solve the ketosis problem. Although unlikely under modern management practices, Dr. Guard also describes simple starvation resulting in death from hepatic failure of about half of the periparturient cows during a 4-week period in a 300-cow herd. The manager was so concerned about fat dry cows that intake was limited to 5 kg of poor quality grass hay. The dying cows were thin with body condition scores of 2 to 2.5, but had severe hepatic lipidosis. The late dry period is not a time to try to get cows to lose weight! Cows that lose condition during the dry period have higher rates of not only ketosis but also of abomasal displacements, milk fever, and metritis.
Long dry periods per se appear to put cows at increased risk for clinical ketosis whether obesity develops or not. Many individual cows with severe ketosis that may be refractory to routine treatments have been discovered to have preceding dry periods of 3 or more months. I have particularly noticed this to be common in cows used for embryo transfer. The pathophysiology of this phenomenon has not been described, but many practitioners have made the same observation. Body condition scores greater than 4.0 are known to increase the incidence of ketosis (see Appendix 1).
The use of ionophores in close up and lactating cow diets now provides a strong management tool for the prevention of ketosis. The action of these antibiotics is to reduce acetate production and enhance propionate production by rumen bacteria. Because propionate is converted to glucose by the liver, an increase in its supply would diminish the likelihood of hypoglycemia and excessive lipid mobilization from fat stores. Administration of monensin by rumen-controlled release during the periparturient period decreased the incidence of ketosis by 50% and decreased both BHB and NEFA concentrations during this period. Intraruminal controlled release capsules are more effective than when the monensin is added to the feed. In situations where monensin is fed within a ration if dry matter intake decreases, the concentration of monensin may be too low to have the needed effect on the rumen microorganisms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree