Chapter 36 Mastitis, inflammation of the mammary gland, can be clinical or subclinical and can be caused by physical or chemical agents. However, the majority of cases are infectious and usually caused by bacteria. Invasion of the mammary gland by microorganisms is characterized by an increased leukocyte count in the milk, the majority of cells being neutrophils. The definition of mastitis is based on the somatic cell count (SCC) of quarter foremilk. The criteria for definition of mastitis have changed from a somatic cell count threshold of 500,000 cells per mL, with or without the detection of pathogens, set by the International Dairy Federation in 1967, to the current threshold of 100,000 cells per mL (Hamann 2005). However, the acceptable limit for the number of somatic cells in milk may differ depending on the country and the requirements of milk-processing companies. Over 130 different microorganisms have been isolated from bovine mastitic milk samples but Staphylococcus aureus, streptococci and members of the Enterobacteriaceae are among the most common aetiological agents in cows and in other animal species. Following entry to the mammary gland, the organisms establish and multiply. Adherence to mammary epithelium is an important virulence factor for contagious pathogens but is not essential for the pathogenesis of coliform mastitis. The principal cell involved in eliminating bacteria from the mammary gland is the neutrophil. Host factors affecting the level and speed of the neutrophil response to infection are important in determining the outcome and severity of disease. These and other factors affecting the occurrence of mastitis are outlined in Table 36.1. Table 36.1 Bacterial, host and environmental factors influencing the occurrence of mastitis The main clinical types of mastitis are: • Peracute: swelling, pain, heat and abnormal secretion in the mammary gland are accompanied by signs of systemic disturbance such as fever, depression, anorexia, weakness and a rapid, weak pulse. The signs are those of a toxaemia or septicaemia. Gangrenous mastitis is included in this category. • Acute: changes in the mammary gland are similar to those of peracute mastitis but the systemic signs are much less severe. • Subacute: no systemic reaction and the changes in the gland are less marked. • Chronic: there are no systemic signs and very few external signs of change in the udder, but abnormal secretion in the gland occurs intermittently. • Subclinical: the infection in the mammary gland is detectable only by bacterial culture or by tests to demonstrate a high leukocyte count in the milk. There is no obvious change in the appearance of the milk. Staphylococcus aureus is notorious for causing a high percentage (up to 50% of the herd) of subclinical infections in dairy herds with a staphylococcal mastitis problem. Mastitis has the greatest prevalence and economic importance in dairy cows but it can be significant in other domestic animals, particularly in sheep, goats and pigs. Table 36.2 gives the most common aetiological agents in small ruminants, their source and the type of clinical syndrome that is usually caused by each agent. Some of the many microorganisms that can cause bovine mastitis are summarized in Tables 36.3 and 36.4. The source and type of mastitic syndrome is given for each pathogen. Formerly, the contagious pathogens caused the majority of the cases of bovine mastitis. Members of this group of pathogens, particularly S. aureus, are still a major problem in individual herds and in areas or countries where the regulatory bulk milk somatic cell count levels, or those accepted by the milk-processing industry, are less stringent. The implementation of control programmes over the last four decades has meant that the number of clinical cases of mastitis has decreased. In the UK the number of clinical cases has decreased from approximately 150 cases per 100 cows per year in the 1960s to between 47 and 65 cases in 2004–2005 (Wilson & Kingwill 1975, Bradley et al. 2007). The environmental pathogens, Escherichia coli and Streptococcus uberis, now account for a large proportion of clinical cases. Table 36.3 Bovine mastitis: principal aetiological agents, usual source and clinical types Table 36.4 Bovine mastitis: less common aetiological agents, usual source and clinical types Coagulase-negative staphylococci and Corynebacterium bovis are quite commonly isolated from milk samples. Corynebacterium bovis causes a persistent infection of the teat duct epithelium and a mild but significant rise in the leukocyte cell count. Because C. bovis is susceptible to disinfectants used as teat-dips, it has been suggested that the presence or absence of the bacterium could be used to monitor the efficiency of the teat-dipping procedure in a herd. Coagulase-negative staphylococci are of low pathogenicity although some strains isolated from mastitis cases have invasive and toxin-producing ability (Burriel & Dagnall 1997, Anaya-Lopez et al. 2006). This group of organisms may cause problems with increased somatic cell count in herds in which other mastitis pathogens are well controlled. The most frequently isolated species are S. chromogenes and S. hyicus. It has been suggested that the presence of coagulase-negative staphylococci may be advantageous to the host as they tend to occupy attachment sites in the teat duct required by the coagulase-positive, pathogenic staphylococci. Host and pathogen factors as outlined in Table 36.1 are important in influencing the outcome of intramammary infection and the type of clinical syndrome observed. In general, the contagious pathogens cause low-grade chronic infections whereas coliform infection is classically characterized by acute disease, severe clinical signs and more rapid clearance of the pathogen from the mammary gland if the animal survives. The streptococci, S uberis and S. dysgalactiae, usually cause mild clinical signs and may persist longer in the mammary gland than the coliform group of organisms. Selected virulence attributes of the major mastitis pathogens that are likely to be important in the establishment and outcome of infection are summarized in Table 36.5. Affected quarters often have large numbers of Staphylococcus aureus in the milk. The staphylococci are easily transmitted during milking via the teat cups or milkers’ hands. Following entry to the mammary gland, adhesins such as fibrinogen-binding protein and fibronectin-binding protein A are likely to be important in adherence to mammary epithelial cells (Castagliuolo et al. 2006). Staphylococcus aureus strains with enhanced ability to survive within both phagocytes and mammary epithelial cells are more likely to set up infection and to persist in the face of the host immune response and antimicrobial therapy. Biofilm formation is now considered to be a factor in persistent infections also (Melchior et al. 2006). The type of mastitis produced by S. aureus ranges from subclinical to the peracute life-threatening forms, one of which is gangrenous mastitis. Gangrenous mastitis is caused by the action of alpha toxin (alpha haemolysin) that damages blood vessels, resulting in ischaemic coagulative necrosis of adjacent tissue. The affected quarter becomes purplish and cold and will eventually slough, if the animal survives the toxaemia. However, subclinical and chronic forms of mastitis are the most common and economically significant types of S. aureus mastitis worldwide. Both lead to a gradual replacement of secretory tissue with fibrous tissue and a subsequent loss of milk production by the affected quarter. The chronic and subclinical forms of mastitis respond poorly to antimicrobial therapy, the reasons for which include host, pathogen and treatment factors (Barkema et al. 2006). Host factors such as age of cow, cell count and duration of infection influence treatment outcome as does treatment duration. Pathogen factors affecting cure rates include the ability to survive intracellularly, biofilm formation and antimicrobial resistance. Apart from therapeutic considerations, the emergence of MRSA as a cause of mastitis is of public health concern, in particular the emergence of a divergent mecA gene in bovine clones of S. aureus and the possibility that cattle may serve as a source for the emergence of new MRSA strains in humans (Garcia-Alvarez et al. 2011, Shore et al. 2011). Up to 65% of cases of E. coli mastitis occurring during the first two months of lactation are the result of infection acquired during the dry period (Smith et al. 1985). It is thought that the survival and multiplication of E.coli strains within the dry mammary gland is dependent in part on the ability of the pathogen to acquire iron (Hogan & Smith 2003). Escherichia coli and other coliforms can multiply rapidly in quarters with a low cell count. The immune response of the host is a major determinant of the outcome of infection and both the somatic cell count and the speed of recruitment of neutrophils into the mammary gland affect the course and severity of the mastitis episode (Shuster et al. 1996). The inflammatory reaction destroys a large proportion of the bacterial population. When Gram-negative bacteria die and lyse, endotoxin is released from the cell wall. The sudden liberation of endotoxin can result in a severe toxaemia that is life-threatening. Approximately 10% of clinical coliform cases result in severe systemic signs (Hogan & Smith 2003). Unlike infections with contagious pathogens, coliform mastitis tends to be of short duration with E. coli infections during lactation typically lasting less than 10 days (Todhunter et al. 1991). However, there is evidence that this pattern may be changing and persistent infections with E. coli may be becoming more frequent (Bradley & Green 2001). This organism is a commensal of the tonsils, gastrointestinal and genital tract of cattle and is found on the coat and in the environment. Faecally contaminated bedding is an important source of infection but heavily used pasture may carry high levels of the pathogen also. The organism is classified as an environmental pathogen but a specific molecule involved in adherence to bovine mammary epithelial cells has been demonstrated (Almeida et al. 2006). Streptococcus uberis produces exoenzymes including a plasminogen activator which converts plasminogen to plasmin. Plasmin is a protease which in turn can hydrolyse casein to peptides which the organism then uses for growth. Production of a capsule confers resistance to phagocytosis. Only a few infections result in systemic signs of fever and inappetance, with clinical signs usually limited to abnormalities in the milk. Streptococcus dysgalactiae has been classified as both a contagious and environmental pathogen. It is found in the environment of cows as well as colonizing the tonsils and mucosa of the mouth and genital tract. It is able to persist within the mammary gland although the mechanism whereby it survives within mammary epithelial cells is unknown. It is isolated relatively infrequently from clinical and subclinical cases of mastitis, 1.5% and 0.4% respectively in a survey by Bradley et al. (2007), but is associated with ‘summer mastitis’ also.
Mastitis
Epidemiology
Microbial factors
Host factors
Environmental factors
Adherence – particularly important for the contagious pathogens
Iron-binding capacity – particularly important for E. coli
Endotoxin production (Gram-negative organisms)
Antiphagocytic capacity
Survival in the immediate environment of the host
Ability to colonize the teat canal
Age: older cows (>4th lactation) are more susceptible; may be due in part to the speed and efficiency of the neutrophil response
Genetics: considerable differences between and within breeds. There is a negative correlation between level of milk production and resistance to mastitis. Heritability of mastitis resistance is low
Stage of lactation
Presence of teat lesions may predispose to inadequate milking or may harbour mastitis-producing bacteria
Immunological factors, including the efficacy of the phagocytic response and levels of lactoferrin, complement and immunoglobulins
Presence of large numbers of potential pathogens in the immediate environment of the animal, whether housed or at pasture. Although ‘coliform’ mastitis is more frequent in housed cows, S. uberis numbers in heavily used pasture are comparable to numbers detected in contaminated bedding
Management factors including feeding practices. Negative energy balance in high-yielding cows early postpartum may compromise the immune response
Milking shed environment including poor milking technique and hygiene
External trauma such as that arising from rough, muddy approaches to the milking shed or, with ewes, the suckling of large vigorous lambs
Milking-machine malfunction or inadequate design
Clinical Syndromes of Mastitis
Mastitis in domestic animals other than cattle
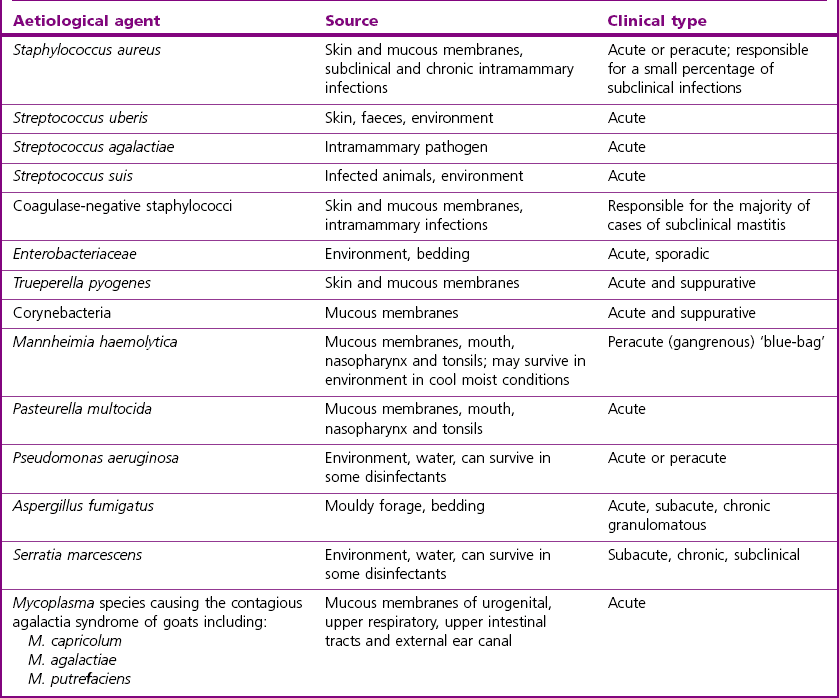
Bovine mastitis
Aetiology
Aetiological agent
Usual source
Clinical type of mastitis
Staphylococcus aureus
Mammary gland of other cows, udder lesions, skin and mucous membranes
Subclinical, chronic, acute and peracute, including gangrenous mastitis. A high percentage of subclinical carriers can occur in a herd
Streptococcus agalactiae
Intramammary in the milk ducts
Acute or chronic with recurring clinical cases. Infection can occur in maiden heifers
S. dysgalactiae
Buccal cavity and genitalia of cattle
Acute
S. uberis
Skin, tonsils, vagina, faeces
Acute, can occur in dry period
Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes
Faeces, sawdust and other bedding. Disease of housed cows
‘Coliform mastitis’. Peracute (toxaemia) usually occurs just after calving in cows with low somatic cell counts. Life-threatening. Acute, chronic and subclinical infections can also occur. Little or no fibrosis in udder of recovered animals
Trueperella pyogenes
Skin and mucous membranes
Peracute, suppurative mastitis
Trueperella pyogenes and Peptoniphilus indolicus (±other organisms)
Both part of normal flora. Infection thought to be fly-borne
‘Summer mastitis’. Most common in dry cows and heifers. Foul-smelling udder secretion. Loss of quarter or death can occur
Aetiological agent
Usual source
Clinical type of mastitis
Streptococcus pyogenes
Human pathogen
Acute mastitis
S. pneumoniae
Human pathogen
Peracute with fever
S. equi subsp. zooepidemicus
Mucous membranes
Subacute or chronic
Enterococcus faecalis
Faeces and skin
Acute mastitis
Pseudomonas aeruginosa
Soil, water or faeces
Peracute (toxaemia) but can be chronic and persistent
Nocardia species
Soil
Sporadic. Acute at first, becoming chronic. Granulomas in udder tissue
Serratia marcescens
Soil and faeces
Peracute (toxaemia) or chronic coliform mastitis
Pasteurella multocida
Mucous membranes (upper respiratory tract)
Acute mastitis
Mannhemia haemolytica
Mucous membranes (upper respiratory tract)
Peracute and severe or acute
Mycoplasma bovis, M. bovigenitalium, other Mycoplasma species
Respiratory tract and mucous membranes
Acute with rapid onset. Most severe in recently calved animals. All quarters often affected. Dramatic drop in milk secretion but rarely any systemic reaction
Mycobacterium bovis
Metastasis from existing tuberculous lesion
Induration and hypertrophy of tissue. Can often palpate lesions in udder after milking
M. fortuitum
M. smegmatis
Soil, but also associated with oil-based intramammary preparations
Severe mastitis. Cows are either culled or die
Fusobacterium necrophorum
Part of normal anaerobic flora of animals
Acute, secretion viscid and stringy. No fibrosis in udder tissue
Bacillus cereus
Associated with feeding brewers’ grains or via intramammary preparations
Peracute or acute
Leptospira serovars Hardjo or Pomona
Water, wet soil or urine of subclinical excretors
Agalactia, self-limiting
Candida albicans (yeast)
Mucocutaneous or environmental
Acute but often self-limiting
Cryptococcus neoformans (yeast)
Often introduced via intramammary tubes
Acute mastitis. Milk is mucoid. Severe swelling of udder
Aspergillus fumigatus (mould)
Often introduced via intramammary tubes
Acute (abscess formation) or chronic
Prototheca zopfii or P. wickerhamii (algae)
Mud, soil, faeces or water. Ubiquitous in environment
Chronic. Very difficult or impossible to treat
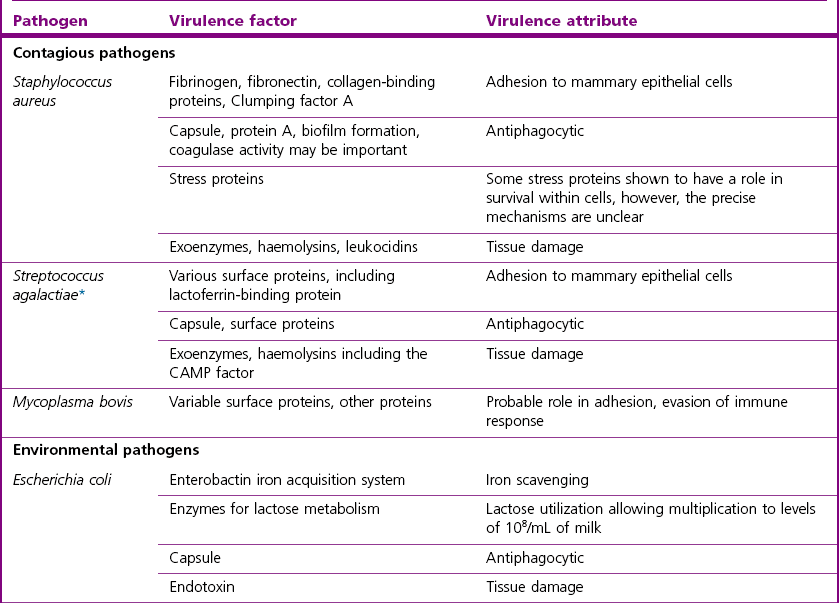
Pathogenesis
Contagious Pathogens
Staphylococcal mastitis
Environmental Mastitis
Coliform mastitis
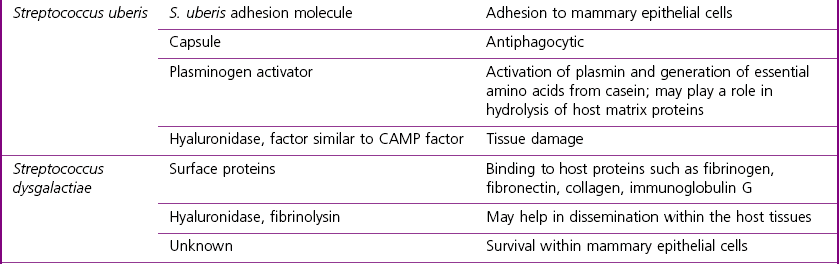
Streptococcus uberis
Streptococcus dysgalactiae
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mastitis
Only gold members can continue reading. Log In or Register to continue