CHAPTER 35 Lyme Disease
ETIOLOGY
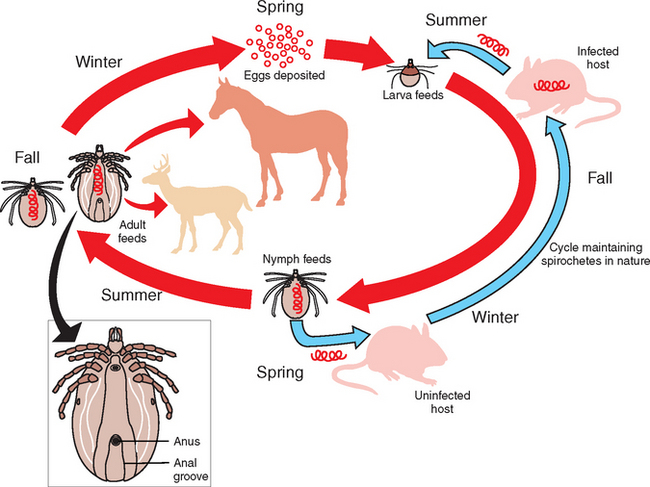
Lyme disease is caused by at least three strains of the spirochete Borrelia burgdoferi sensu lato complex,1,2 which includes several worldwide species. The North American strain is B. burgdorferi sensu stricto (“in the strict sense”).3 B. burgdorferi organisms are helical-shaped, gram-negative, unicellular spirochetes with flagellar projections.4 B. burgdorferi bacteria are not free-living organisms and quickly die outside a host. They are maintained in a 2-year enzootic life cycle that involves ixodid ticks (in North America) and mammals.4 Deer and the white-footed mouse are the most common mammals involved in maintaining the life cycle of this spirochete (Fig. 35-1).
EPIDEMIOLOGY
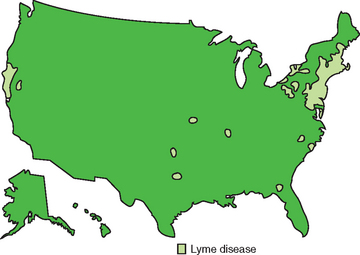
The seroprevalence of Lyme disease in horses in the United States is not known but is likely similar to that in humans (Fig. 35-2). The mid-Atlantic and northeastern states have a high seroprevalence, as do areas of Minnesota and Wisconsin. Seropositive horses are rare in the Rocky Mountain states, the Dakotas, and Nebraska. In one New England survey, 45% of horses had Borrelia antibodies.12 In a Wisconsin study, 118 of 190 horses were serologically positive.13
PATHOGENESIS
Infection in horses is caused by attachment and prolonged (>24 hours) feeding of infected adult Ixodes spp., ticks. Female ticks are likely the competent vector and can be identified by the complete arch over the anus (see Fig. 35-1). Larvae and especially nymphs are responsible for a high percentage of infections in humans because they are small and often escape visual inspection.5 It is not known if these stages transmit the spirochete to horses. Once feeding begins, the organism begins its complicated up-and-down regulation of genes to enhance survival in the host. Exact pathogenesis of Borrelia in the horse is not known. After experimental infection of ponies, the organism appears to reside mostly in skin near the tick bite, as well as in connective tissue and muscle and around nerves and blood vessels near synovial membranes.14 A lymphocytic plasmacytic reaction may occur within these tissues, and in experimentally infected ponies, this reaction was associated with the highest concentration of the Borrelia organism.14,15
The organism lives in the tick gut and is transferred to animals during blood meals. Generally, 24 to 48 hours of attachment is required to transfer the organism successfully from the tick to the mammalian host.5 This time may be needed for the organism to downregulate an outer membrane protein (OspA), which may be important to maintain survival in the mammalian host.6 Conversely, other surface proteins (e.g., OspE, OspF) that are in low concentration in the tick gut are upregulated to enhance complement resistance and other methods of immune evasion in the mammalian host.7 The changes in expression of surface proteins may be triggered by the blood meal. Other genes permit antigenic variation, ensuring survival in the host. Many other components of the agent may be important for infection or virulence, but these are poorly understood.8 Borrelia burgdoferi may also survive in the host by residing in collagen and connective tissue, having no requirement for iron and by possibly forming resistant cysts within the host.9–11
CLINICAL FINDINGS
A wide variety of clinical signs have been attributed to Borrelia infection in horses, but cause and effect have been difficult to document. Clinical signs most often attributed to equine Lyme disease include low-grade fever, stiffness and lameness in more than one limb, muscle tenderness, hyperesthesia, lethargy, and behavioral changes.21–25 Unlike human Lyme disease, joint effusion has been minimal in most Lyme-suspect horses, although it was pronounced in one horse. Muscle wasting and pain over the thoracolumbar area have been present in a few horses with high serum titers. One suspect horse the author examined had ataxia and severe lymphocytic infiltration of the meninges. There is one published report of neurologic dysfunction in a horse attributed to Lyme disease,22 and in another report, panuveitis was reported in a pony.26 There has been no further evidence to suggest that Borrelia infections are in any way associated with recurrent uveitis in the horse. The high fever and limb edema typically reported in association with Borrelia seroconversion are most often the result of Anaplasma phagocytophila infection, because many ticks are concomitantly infected with both Borrelia and A. phagocytophila.27
DIAGNOSIS
The diagnosis of exposure to Borrelia is straightforward, but determining if the horse is currently infected is more difficult, and to determine whether or not clinical disease is associated with Borrelia is extremely difficult. Enzyme-linked immunosorbent assay (ELISA, Cornell Diagnostic Laboratory, Ithaca, New York) or immunofluorescent antibody (IFA) testing is the preferred screening test for detection of antibodies indicating exposure. If the ELISA value is less than 110 units, it indicates nonexposure, distant exposure but no current infection, or recent infection (within 2 months).14 A repeat sample taken in 2 months that remains less than 110 would rule out the latter.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree