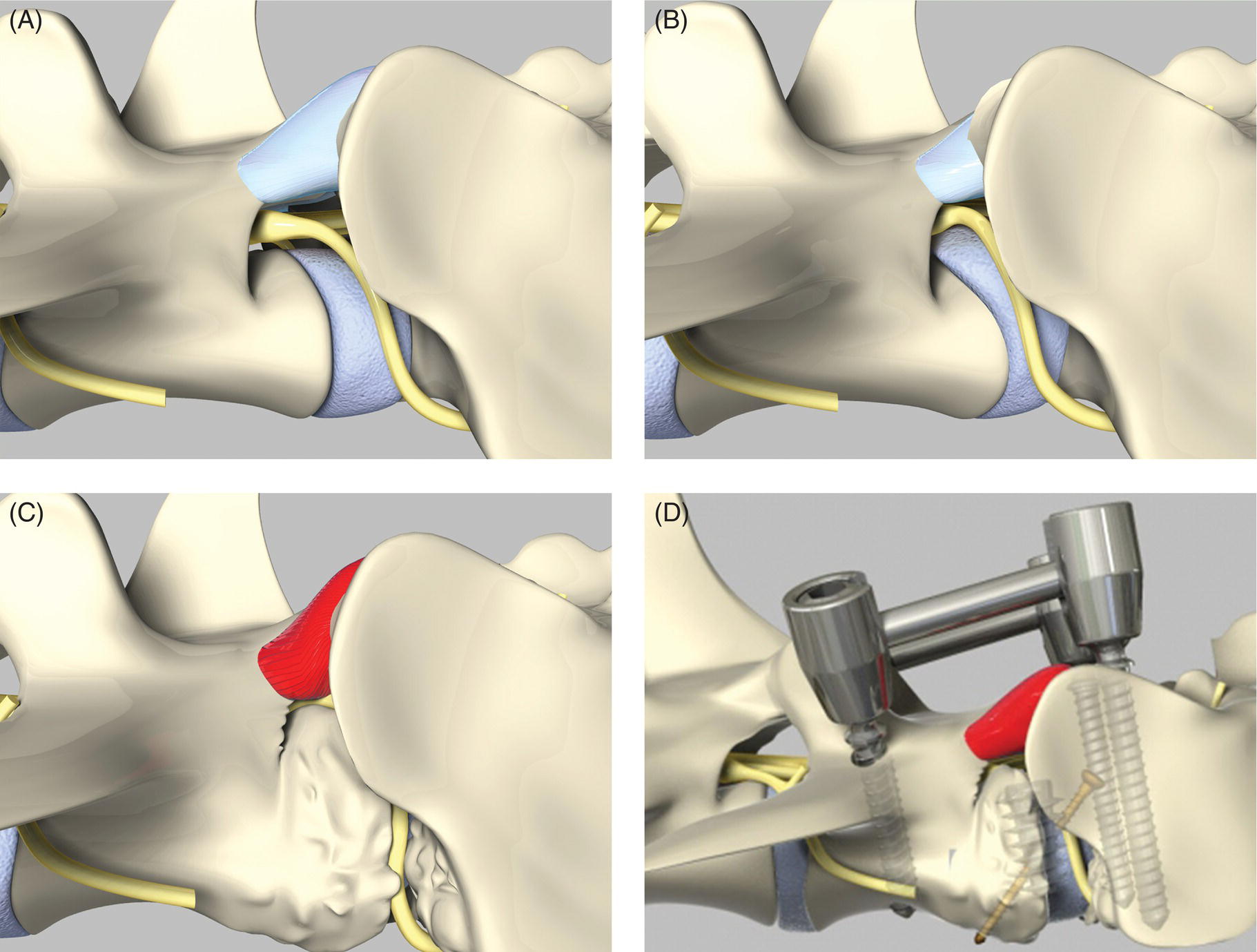
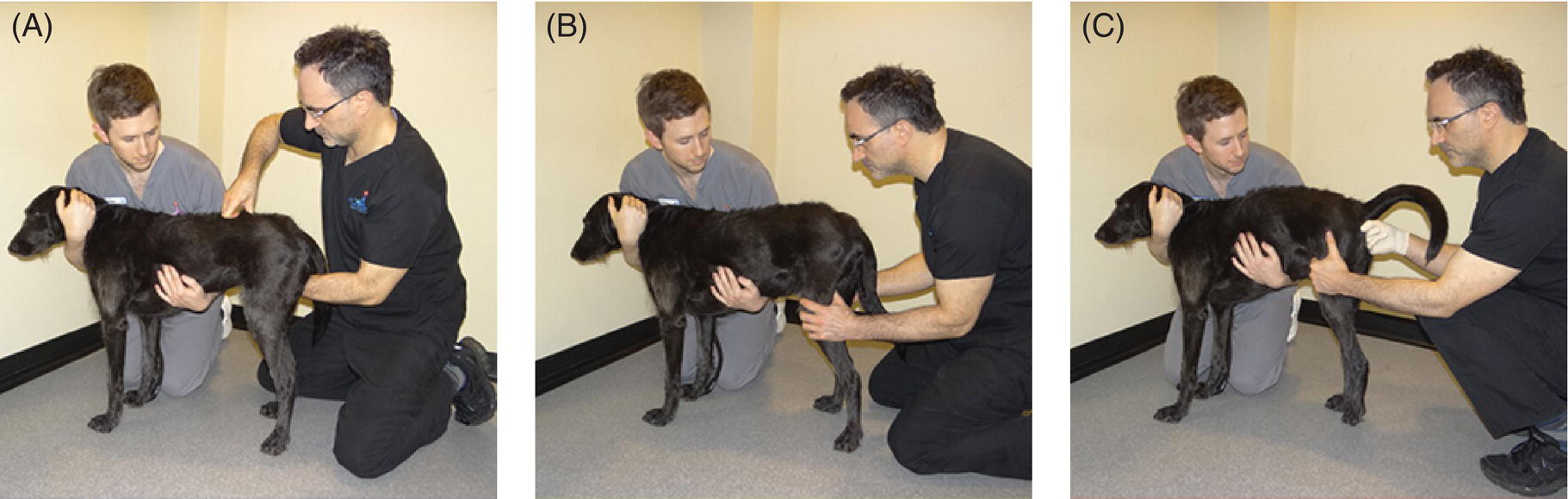
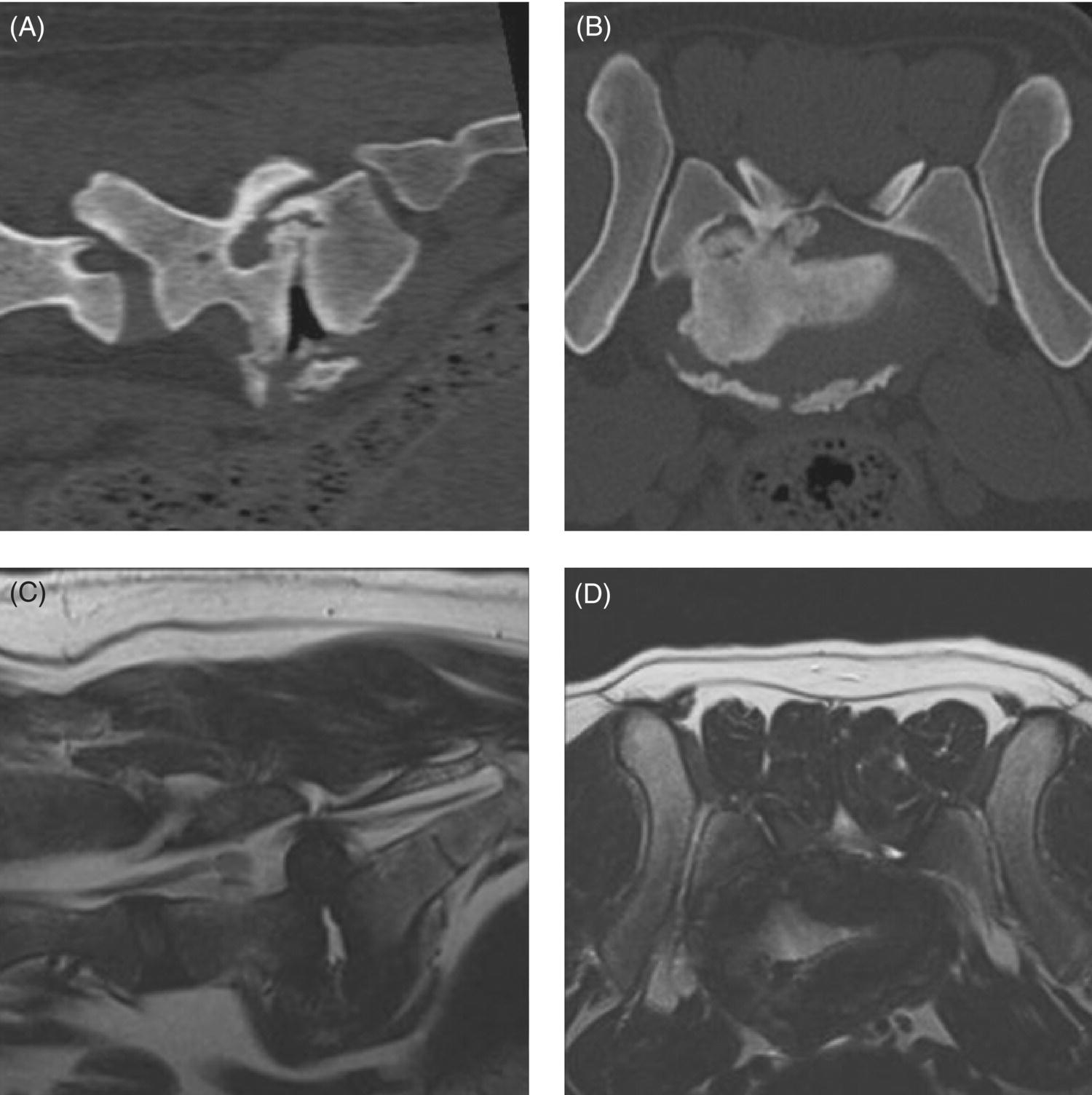
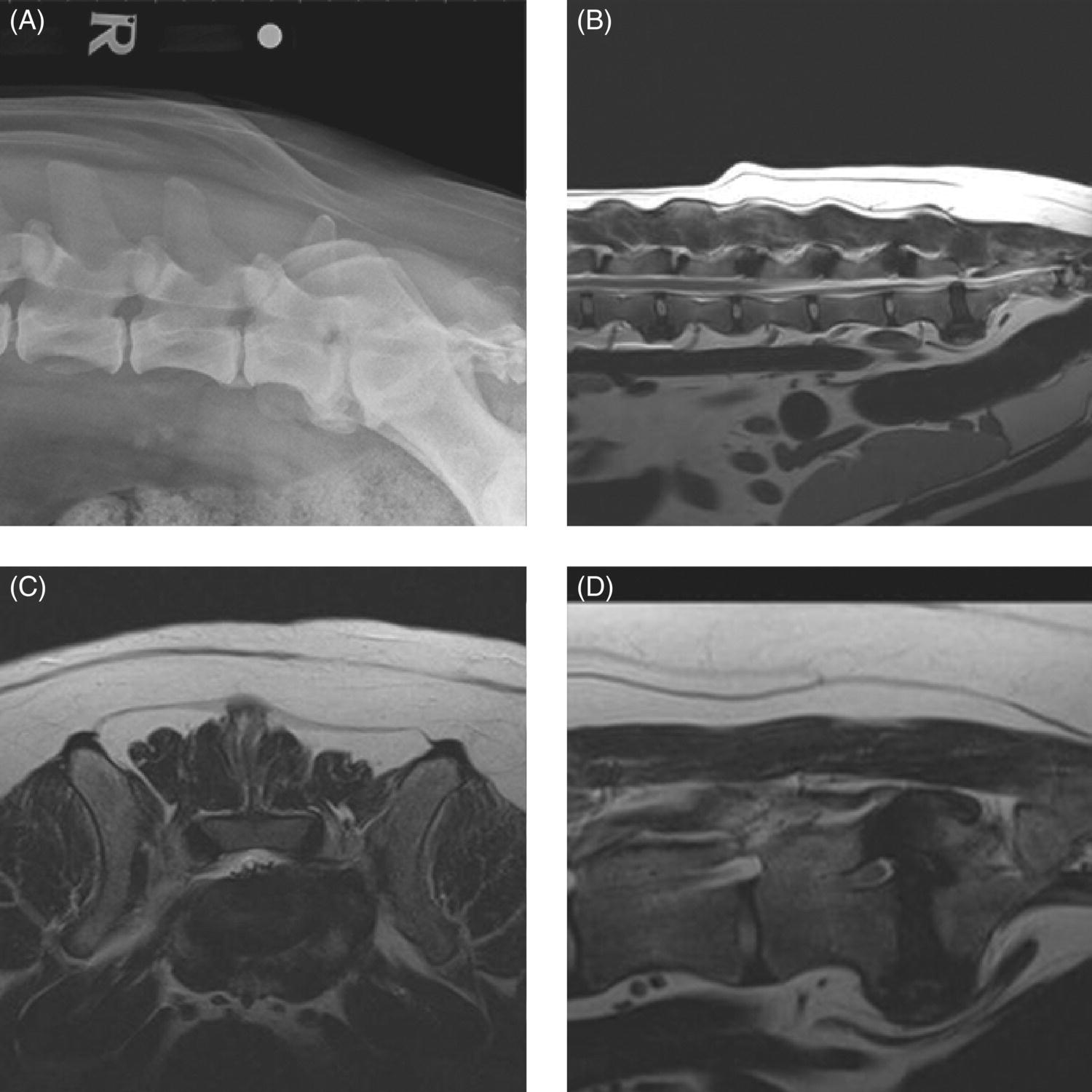
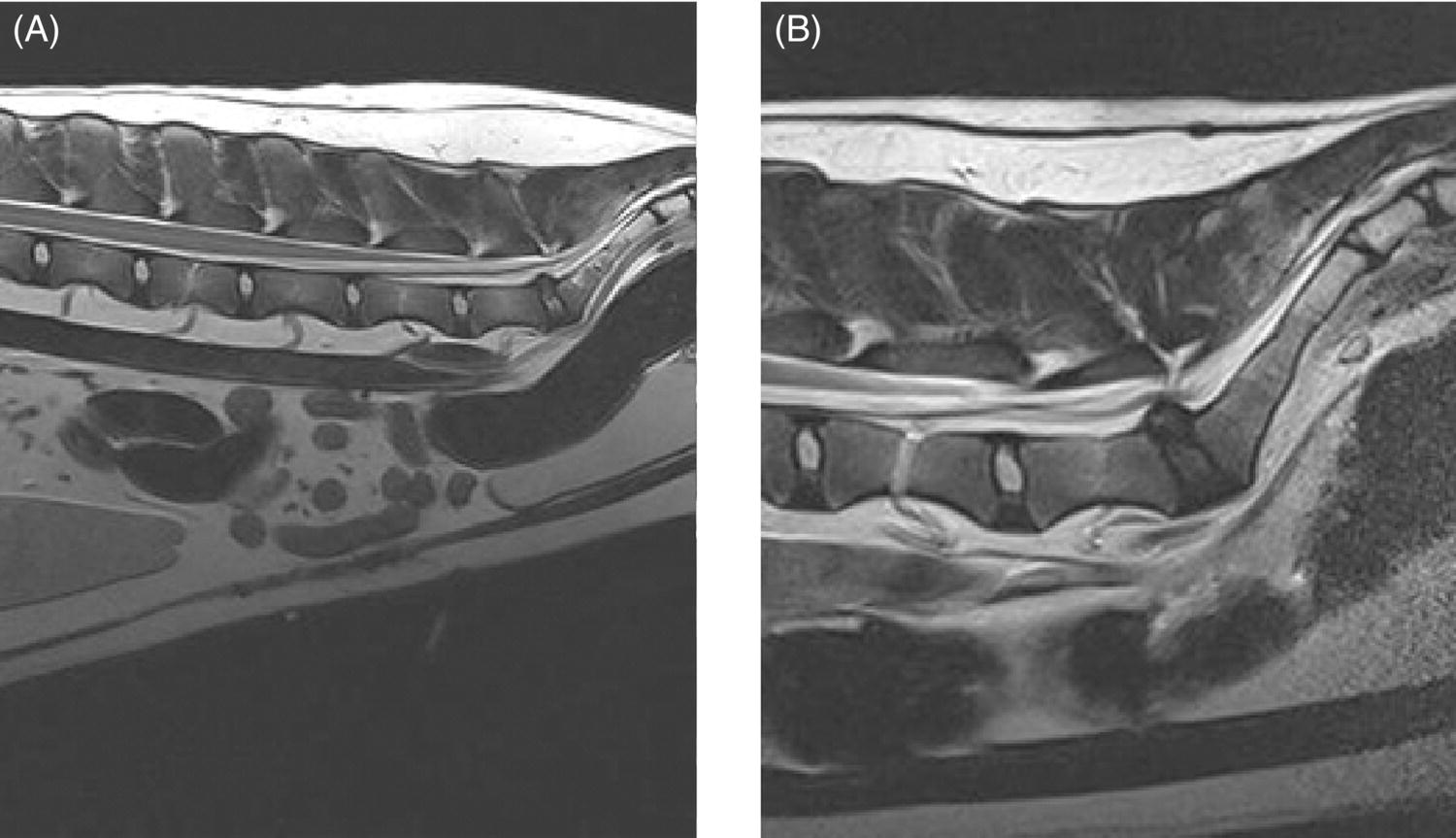
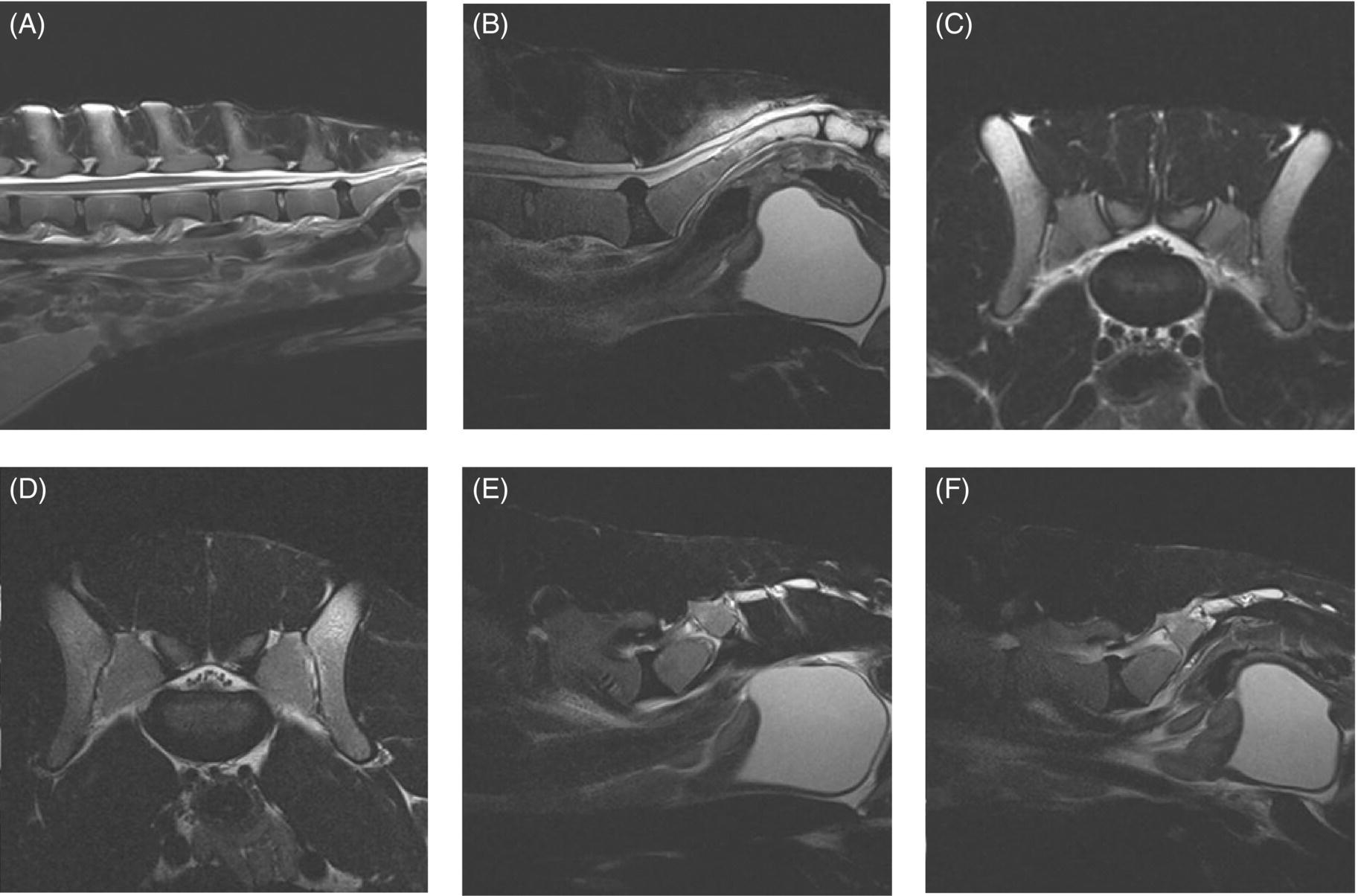
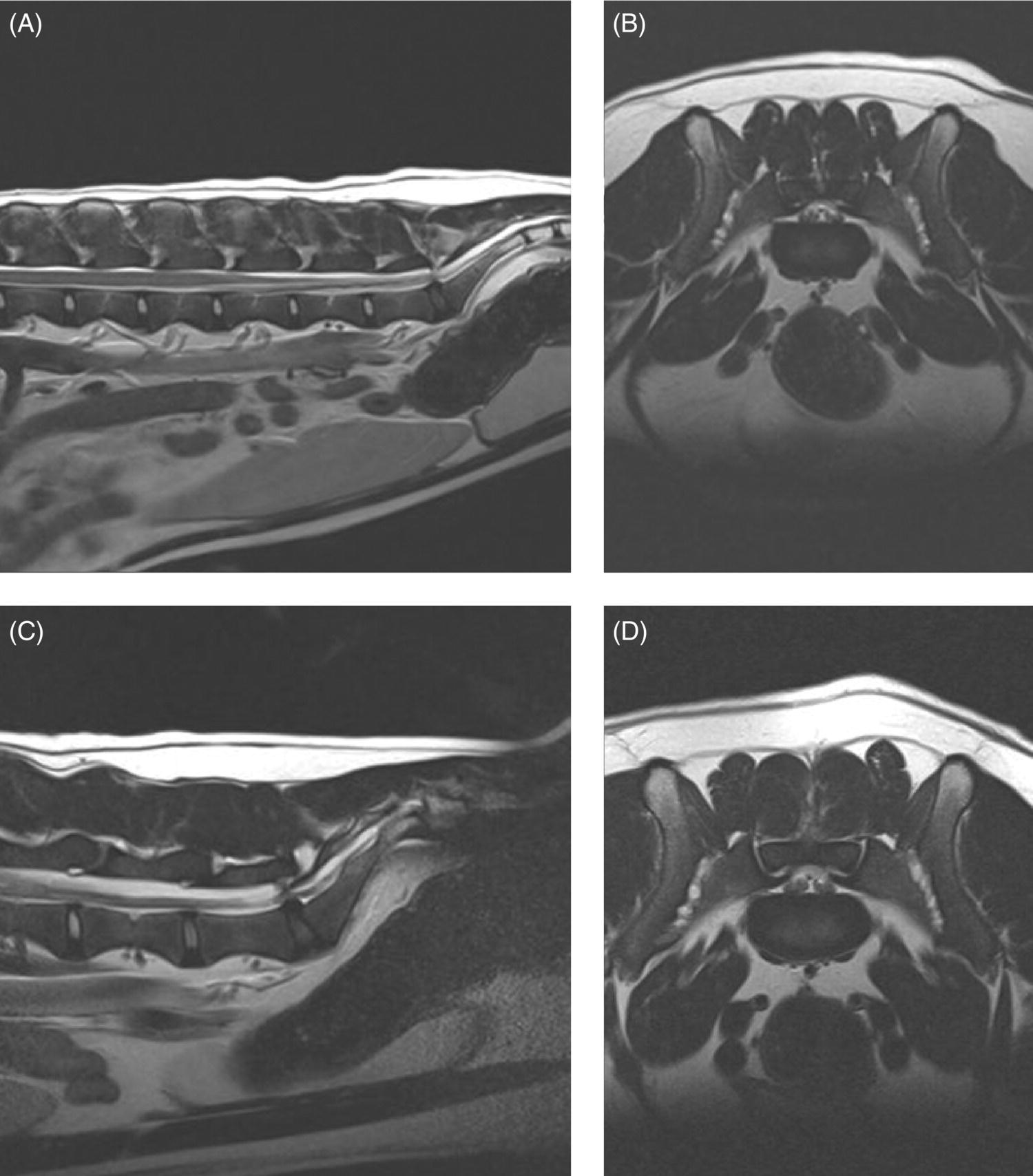
32 Michael Farrell and Noel Fitzpatrick Canine lumbosacral (LS) disease is an umbrella term that refers to any condition causing compression of the cauda equina or its regional blood supply [1]. Although neoplasia, discospondylitis, sacral osteochondrosis, and trauma have been reported as possible causes of canine LS disease, the degenerative form is by far the most common [2]. Degenerative lumbosacral stenosis (DLSS) is a multifactorial disorder characterized by varying combinations of Hansen type II intervertebral disc (IVD) protrusion (or less commonly type I extrusion), hypertrophied soft tissue (ligamentous and synovial structures), articular facet joint osteophytosis, LS spondylosis, and instability [3, 4]. Clinical signs are a consequence of direct compression of the cauda equina, impingement of the spinal nerve roots as they exit their respective foramina, or a combination of both [5–7] (Figure 32.1A, B, and C). Figure 32.1 Schematic drawings of lumbosacral intervertebral disc degeneration. (A) Normal, (B) central and abaxial (lateral) intervertebral disc protrusion, (C) vertebral end-plate spondylosis and articular facet inflammation compressing the L7 nerve root in addition to disc protrusion, (D) placement of the Fitz Intervertebral Traction Screw (FITS) device through a dorsal laminectomy facilitates distraction of the end plates such that compression on the L7 nerve root(s) is alleviated. Stabilization is then provided using screws in the vertebral bodies of L7 and the sacrum connected by clamps and rods dorsally (Fitzateur). Source: Images by Tim Vojt, The Ohio State University. © Tom Vopjt. The cauda equina lies within the LS canal and is composed of the seventh lumbar (L7), the sacral, and the caudal nerve roots [8]. The LS nerve roots and associated dorsal root ganglia exit through the lateral lumbar vertebral canal, which is divided into the entrance zone (lateral recess), middle zone, and exit zone [9]. Substantial epidural fat is normally present in the LS canal, and this may allow for a certain “anatomical reserve” of compression to occur before neurological deficits are seen [10]. When this reserve becomes exhausted, clinical signs relate either to sciatic neurapraxia (proprioceptive deficits, decreased hock flexion, patellar hyperreflexia, and sometimes flaccidity of the caudal thigh musculature) or to the pelvic, pudendal, or caudal nerves (urinary or fecal incontinence, motor or sensory deficits to the perineum or tail) [11]. It is noteworthy that in the authors’ experience, pain only or pain and lameness are often the only signs manifested. Pain may be elicited on physical examination by application of direct pressure over the lumbosacral junction, pressure application to the pathway of the sciatic nerve in the caudal recess of the thigh musculature, extension of the LS joint, or per rectal palpation of the sciatic nerve pathway (Figure 32.2). Figure 32.2 Clinical examination for lumbosacral-associated pain. (A) Application of digital pressure to the dorsal aspect of the lumbosacral spine. (B) Application of digital pressure to the pathway of the sciatic nerve in the groove between the semitendinosus/semimembranosus and the biceps femoris on the caudal aspect of the thigh. (C) Application of digital pressure per rectum to the sciatic nerve as it courses over the lesser ischiatic notch on the axial aspect of the pelvis. The etiology of DLSS remains controversial. Although neither the heritability nor the mechanism of inheritance for DLSS has been established to date, the high prevalence in German shepherd dogs (GSD) suggests a hereditary predisposition. The complex multifactorial pathogenesis supports a polygenic etiology with potentially important environmental influences. Previous studies have investigated the influence of anatomical variations peculiar to the GSD on the pathogenesis of DLSS, including a variant anatomy of the LS facet joints and a relatively high incidence of lumbar transitional vertebrae in this breed [12–15]. It has been hypothesized that these differences in vertebral morphology may alter motion of the LS discovertebral segment, resulting in excessive loading of the IVD, which ultimately leads to disc degeneration [13]. It is also possible that the high incidence of DLSS in military working dogs might indicate that strenuous physical activity influences phenotypic expression of DLSS in genetically susceptible individuals [16]. Whether vertebral stabilization constitutes the most appropriate treatment option for DLSS depends in part on whether LS instability is the primary pathophysiological defect. It is thought that age-related degeneration of the nucleus pulposus through a progressive loss of its hydrodynamic properties is the most likely origin of lumbar spinal instability [17, 18]. The pathological consequences of this instability are degeneration of other stabilizing structures, including loss of pretension in the ligamentum flavum and longitudinal ligaments, laxity and inflammation of the facet joint capsule, and subluxation of the facet joint [18]. Other sequelae include thickening of the cartilaginous vertebral end plates and development of periarticular osseous proliferations, such as facet joint osteophytes and spondylosis [19]. This process further impairs the nutritional supply to the IVD, triggering a negative spiral leading to structural failure of the disc [20, 21] (Figures 32.1, 32.3, and 32.4). A pathophysiological hypothesis involving progressive instability is supported by MRI evidence of these compensatory changes in clinically affected dogs [10] and by ex vivo biomechanical [15] and in vivo kinematic [22] studies demonstrating significantly different mobility of the LS spinal segment in dogs predisposed to DLSS because of their breed (GSD) or dogs affected by radiographic DLSS when compared with control populations of low-risk [15] or unaffected [22] dogs. Figure 32.3 CT and MRI scans of the lumbosacral spine of a nine-and-a-half-year-old Rhodesian ridgeback dog. (A and B) CT scans in parasagittal and transverse planes illustrate dramatic spondylotic new bone formation encroaching the L7 neuroforamen on the right (left of picture). (C and D) T2-weighted MRI scans in sagittal and transverse planes illustrate severe intervertebral disc degeneration at the lumbosacral junction and very severe compression of the cauda equina and the abaxial nerve roots by protruding disc material and spondylotic new bone. The right L7 neuroforamen (left of picture) is most significantly compressed. Figure 32.4 Radiographic and T2-weighted MR images of the lumbosacral spine of a 7-year-old German shepherd dog. (A) Radiography in lateral projection demonstrates ventral and abaxial spondylosis. (B) Midsagittal plane MRI demonstrates intervertebral disc degeneration at the lumbosacral junction associated with central protrusion and ventral spondylosis. (C and D) Transverse and parasagittal plane MR images demonstrate marked ventral spondylosis and marked abaxial encroachment of the left L7 neuroforamen (right on image) by protruding disc material and new bone formation. The right-sided neuroforamen is encroached to a lesser extent. Some dogs affected by DLSS manifest disc-associated compression of the cauda equina or spinal nerve roots only when the LS spinal segment is fully extended [23]. This phenomenon is commonly termed “dynamic” LS IVD compression and is exemplified by the exaggerated pain response in some dogs during the lordosis test (which involves isolated hyperextension of the LS spine) and variable LS IVD protrusion noted during “dynamic” (flexion–extension) myelography or MRI [10, 24, 25] (Figure 32.5). MRI has rapidly become the gold standard diagnostic modality, and the authors have frequently observed lateral IVD protrusion, which can occur in the absence of central disc herniation (possibly similar to low lumbar disc disease seen in humans (see Chapter 3)). This is most common in highly active dogs such as those involved in agility or similar working/sport pursuits and can result in pain or unilateral lameness only. In some cases, the authors have observed that the cranial extent of the dorsal lamina of the sacrum may extend ventral to the caudal extent of the dorsal lamina of L7 and impinge the dorsal aspect of the cauda equina in hyperextension (Figures 32.6 and 32.7). This may be similar as well to the syndrome of spondylolisthesis reported in some human patients with LS disease (see Chapter 3). Figure 32.5 T2-weighted MRI scans of the lumbosacral spine in midsagittal plane of a five-and-a-half-year-old collie dog. (A) Neutral position MRI showing intervertebral disc degeneration at the lumbosacral junction and mild protrusion. (B) Hyperextended position MRI showing intervertebral disc degeneration at the lumbosacral junction and significant protrusion. This constitutes a dynamic intervertebral disc protrusion. Figure 32.6 T2-weighted MRI scans of the lumbosacral spine of a 7-year-old mixed breed dog. (A) Neutral position midsagittal plane showing intervertebral disc degeneration at the lumbosacral junction and mild protrusion. (B) Hyperextended position midsagittal plane showing intervertebral disc degeneration at the lumbosacral junction and significant dynamic protrusion. (C and D) Transverse plane images of (A) and (B), respectively, showing central protrusion in neutral position and central plus abaxial protrusion in hyperextended position, with associated encroachment of the L7 neural pathways. (E and F) Parasagittal left and right images of the hyperextended position shown in (B) and (D) demonstrating encroachment of the L7 neuroforamina by disc annulus and migration of the dorsal lamina of S1 ventral to that of L7, creating an “hourglass” dorsal and ventral compression of the L7 nerve root. Figure 32.7 T2-weighted MRI scans of the lumbosacral junction of a 7-year-old border collie dog in midsagittal and transverse planes. (A and B) Neutral position showing a degenerated lumbosacral disc with mild protrusion centrally on sagittal images and abaxial protrusion, worse on one side, on transverse image. (C and D) Hyperextended position manifests greater protrusion of the degenerated lumbosacral disc and ventral migration of the dorsal lamina of the sacrum relative to L7, creating “hourglass” compression of the cauda equina and greater abaxial compression of the L7 neuroforamina. It is also important to recognize that any pathological LS instability is superimposed on an articulation that is already considered high motion. The healthy canine L7–S1 segment has a greater mobility in flexion and extension than the other lumbar segments [15, 25–27]. Although the most prominent motion direction is flexion and extension, lateral bending and torsion are also possible, and increased ranges of rotational and shear motion have also been reported within the normal LS discovertebral segment in comparison with the other lumbar spinal segments [18].
Lumbosacral Disc Disease: Is Vertebral Stabilization Indicated?
Introduction
Functional anatomy and clinical signs
Etiology
Pathogenesis
Treatment options
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


