CHAPTER 5 Lower Respiratory Tract
Signs of lower respiratory tract disease include dyspnea, tachypnea, coughing, and stridor. A patient may expel mucus, exudate, or blood while coughing. If the respiratory disease is caused by an infectious agent, the horse may have a fever. Auscultation may reveal rales of various types, including crackles, wheezes, rhonchi, and pleural friction rubs. After the initial clinical examination of a patient with respiratory disease, a clinician may examine the patient in a variety of ways. With small animals the next phase is usually radiographic examination, but with larger animals radiographic examination may be difficult because of the relative lack of powerful radiographic equipment necessary for quality radiographic examination. However, quality radiographic examination should be conducted on horses with respiratory disease when possible.1
Pleural effusion may accompany conditions, especially infectious diseases, affecting the lower respiratory tract. Therefore signs referable to pleural effusion may also be found, and cytologic evaluation of pleural fluid may be indicated (see Chapter 8). Other general health examinations, such as hematologic and serum biochemical examinations, are also indicated in horses with respiratory disease. Specific examinations of the lower respiratory tract include bronchoscopy and laboratory evaluation of material from the lower respiratory tract. Laboratory examinations of this material include culture and microscopic examination. Material from the lower respiratory tract is usually obtained by transtracheal wash (TTW), bronchoalveolar lavage (BAL), and occasionally transthoracic aspiration.1,2
Sample Collection
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) is usually performed during bronchoscopic examination of the lower respiratory tract. Fiberoptic endoscopy allows detailed examination and sampling of specific locations. The endoscope or catheter is passed into a major bronchus. Usually it is necessary to sedate the patient with xylazine (0.5 mg/kg body weight). Lidocaine (up to 60 ml of 0.5% lidocaine) is often infused through the endoscope to desensitize the airways.3 After the endoscope is gently wedged in a small bronchus, 30 to 250 ml of sterile saline (without bacteriostatic preservative) is infused and as much as possible is immediately retrieved by aspiration with syringes or a suction pump.3,4 In people, multiple infusions and aspirations have been used to obtain samples from the bronchioli and alveoli. The initial washing contains material primarily from the small airways, while subsequent washings contain material primarily from the alveoli.5 Greater detail of BAL technique is described elsewhere.3
Tracheal Wash
Tracheal wash or lavage may be performed during endoscopic examination. Tracheal wash, however, is more frequently performed by the transtracheal method. With the transtracheal wash (TTW) technique, a catheter is passed through the skin, between tracheal rings, and into the tracheal lumen. The TTW technique is performed by first clipping approximately a 10-cm2 area in the region of the upper trachea, performing a surgical scrub, and anesthetizing the skin with a local anesthetic, such as 2% lidocaine. When the area is sufficiently anesthetized, a small stab incision is made through the skin. An intravenous cannula (eg, Medicut, Sherwood Medical, St. Louis, MO) or a large-bore hypodermic needle is inserted between two tracheal rings into the tracheal lumen. While the cannula is directed caudally, a sterile polypropylene catheter is passed approximately to the area of the tracheal bifurcation. Immediately after 30 to 60 ml of sterile saline (without bacteriostatic preservative) is infused, as much as possible is rapidly aspirated. Alternatively, the saline may be aspirated intermittently while withdrawing the catheter. It has been suggested that either gentle exercising or provoking a cough in a patient before sample collection helps obtain a representative sample.3,6,7
After the sample is collected, the catheter should be withdrawn and part of the sample readied for culture if culture is indicated by clinical signs or the gross appearance of the aspirated material (see Chapter 1). The remainder of the sample is retained for subsequent analysis. A small amount of antiseptic solution should be applied to the skin incision after the catheter and cannula are withdrawn.3,6–9
Complications: Complications of TTW are rare, but occasionally subcutaneous, peritracheal, and mediastinal emphysema may occur. Subcutaneous infection from leakage of exudate or from external contamination has been reported.8 Rarely the cannula can sever the catheter when the catheter is withdrawn. This can be prevented by withdrawing the cannula or needle from the trachea before retrieving the catheter.3 It is reported that horses quickly expel the severed end of the catheter by cough.3,9
Other Methods
Techniques for obtaining specimens with endoscopes and guarded brushes or protected aspiration catheters have been described. Such sample are uncontaminated by bacteria from the mouth or nasal passages of the upper airways.10–12 The use of these methods for obtaining material for cytology evaluation has not been evaluated but potentially could provide cellular material from very localized lesions detected during bronchoscopic evaluation.
Percutaneous transthoracic biopsy is occasionally used to obtain biopsies of lungs and potentially could be used for fine-needle aspirates. A survey of large animal diplomates of the American College of Veterinary Internal Medicine indicated that sampling lung tissue in this way is useful for diagnosis of a variety of conditions, including those that produce a miliary pattern, suspicion for pulmonary infiltrative disease, pulmonary neoplasia, and pulmonary abscessation.2
Total and Differential Cell Counts
There is moderate to marked variation in the amount of fluid recovered after BAL or TTW procedures. The variation is less in BAL, however. Some investigators have suggested that total and differential cell counts may be of particular value for evaluating BAL fluid, but this is not commonly done in routine evaluation of BAL fluid.4,8,13–15 Probably because different amounts of fluid have been infused in studies evaluating BAL cellularity, marked differences in total cell count have been reported.16–19 When cell counts are determined, a hemacytometer technique is used. The sample dilution should be varied based on the estimated cellularity or the gross appearance of the fluid. One group suggested a 1:2 dilution for clear samples and up to 1:21 dilution for more turbid samples.8 Differential cell counts may be of greater value than total cell counts. Cells are classified as epithelial cells, macrophages, neutrophils, lymphocytes, eosinophils, mast cells, and other cells (eg, squamous cells). Epithelial cells may be further divided into columnar, cuboidal, and goblet cells.16–19
Staining Smears
Air-dried smears are usually stained by Romanowsky methods (Wright’s, Giemsa, May-Grünwald Giemsa, etc). Hematoxylin and eosin, Sano’s trichrome, and Papanicolaou stains also have been used.8,17,20,21 Other stains, such as Gram stain to identify bacteria (especially gram-positive bacteria), PAS stain to define fungi, and Perl’s Prussian blue or Gomori’s stains for iron, are also useful.17
Microscopic Features
Elements in BAL and TTW Fluid
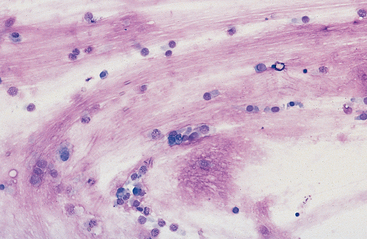
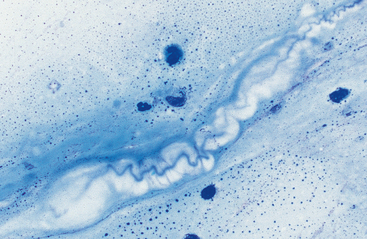
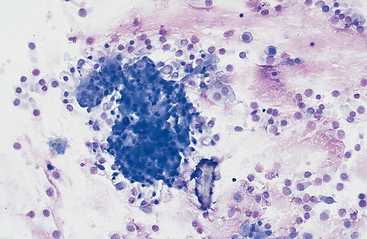
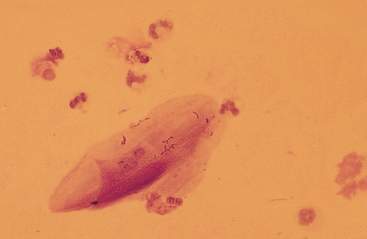
Mucus: Material obtained from the lower respiratory tract always contains some mucus.17 In the collected fluid, mucus appears as strands of flocculent material. In Wright’s-stained slides, mucus appears as pink to light blue amorphous strands (Fig. 5-1). Also, granular structures of similar staining quality sometimes are seen. These are mucus particles that have recently been released from goblet cells (Fig. 5-2). Mucus may also appear as dark-staining tight spirals (Curschmann’s spirals), which are inspissated mucous casts of small bronchioli (Fig. 5-3). Curschmann’s spirals are usually found in samples from animals with prolonged and excessive production of mucus.
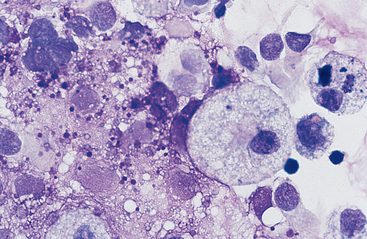
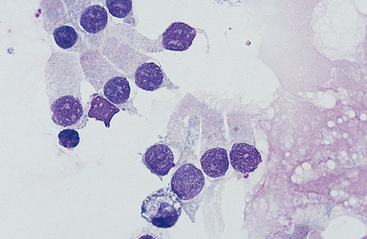
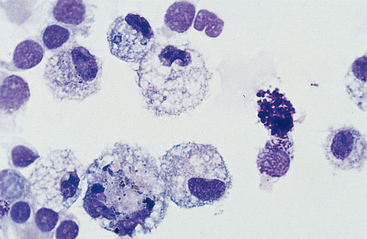
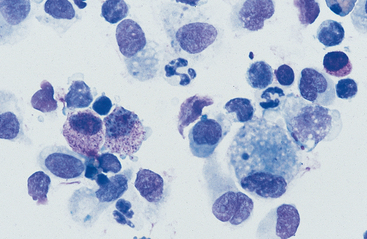
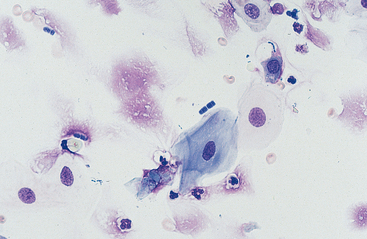
Cells: Cells from the lower respiratory tract include epithelial cells, resident macrophages, and inflammatory cells.17 Epithelial cells are of various types, depending on their origin. They consist of ciliated columnar epithelial cells, nonciliated epithelial cells of various types, and goblet cells (Figs. 5-4 and 5-5). Ciliated columnar epithelial cells are elongated and may have distinct, fine cilia at the end of the cell opposite the nucleus. Goblet cells are similar in shape to columnar epithelial cells, but they do not have cilia, and with some stains they may contain numerous azurophilic granules of mucus. Frequently, groups of basophilic epithelial cells are found in material from the lower respiratory tract. Usually these are adhered clusters of hyperplastic epithelial cells that have been stimulated by local irritation, especially with conditions that cause chronic inflammation (Fig. 5-6).
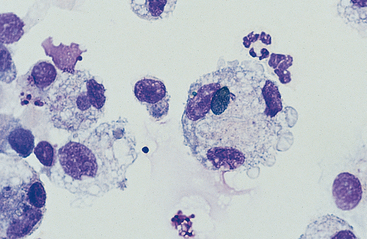
Macrophages are resident cells of alveoli. They may transform into reactive inflammatory cells. Inflammatory cells include neutrophils, reactive macrophages, and eosinophils. Lymphocytes and mast cells are also found in lower respiratory samples from horses (Fig. 5-7). Erythrocytes are usually present, most frequently because of minor trauma of the epithelium that occurs during TTW or BAL, but they may also accompany inflammatory conditions or conditions that cause hemorrhage, such as exercise-induced pulmonary hemorrhage (EIPH).17
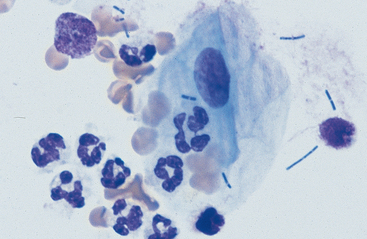
Substances from outside the lower respiratory tract also may be found. Usually these are the result of contamination during the TTW or BAL procedure, but they may be inhaled material that has not been expelled by the mucociliary action of the respiratory epithelium. Frequently, squamous epithelial cells or squamous-cell particles from the mouth or pharynx contaminate the sample (Figs. 5-8 and 5-9). Squamous cells are large cells with a distinctly flattened appearance. They may appear folded or rolled up. Usually they stain moderately basophilic, but occasionally they are slightly acidophilic. Their nuclei are often pyknotic or fragmented. Bacteria may be adhered to their surfaces. These organisms are contaminants from the mouth, pharynx, or nasal passages (Fig. 5-9).
Various other contaminants may be found in material obtained from the lower respiratory tract. These include some bacteria, plant material (pollen), fungal elements, and crystals (Figs. 5-8 to 5-12). Most of these substances are usually contaminants. Bacteria may be either pathogens or contaminants obtained from the upper airways, the mouth, or the environment. Cytologic evaluation and the associated clinical signs are used to determine the significance of bacteria or the other elements. Granules of surgical glove powder (starch granules) (Fig. 5-9) are also found. Starch granules are light to moderate blue and round or imperfectly hexagonal. They have a refractile zone in their center. Microscopically, starch granules have a three-dimensional character. The depth dimension can be appreciated by observing the granules coming into focus in a different plane from the flattened cells on a slide. Other crystalline structures are also found occasionally. These crystals may be free or within macrophages. Generally they are clear and slightl y refractile with straight borders and distinctly angular corners.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree