CHAPTER 5 Lower Motor Neuron
Spinal Nerve, General Somatic Efferent System
LOWER MOTOR NEURON
The general somatic efferent system of the lower motor neuron includes the neurons that innervate striated voluntary skeletal muscle that is derived from somites and somatic mesoderm in the body wall’s limb buds and from somitomeres in the head. These neurons are located in all of the spinal nerves and all of the cranial nerves except I, II, and VIII. This chapter describes the spinal nerve GSE-LMN. Chapter 6 describes the GSE-LMN present in cranial nerves.
SPINAL NERVE: GSE-LMN
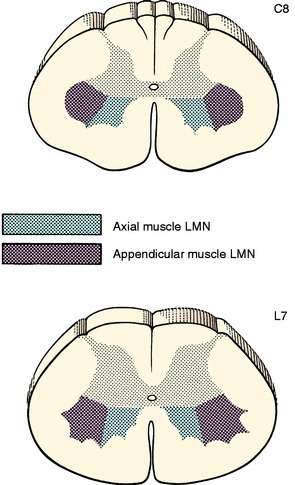
The neuronal cell bodies of this system are located in the ventral gray columns throughout the entire spinal cord. The shape and size of the ventral gray column reflect the number of neurons present, which is determined by the volume of striated muscle that is innervated. The GSE neurons innervating the axial muscles populate the medial portion of the column. Those innervating the appendicular muscles are located laterally and cause the lateral bulge of the ventral gray column that is evident at the cervical and lumbosacral intumescences (Fig. 5-1). These lateral portions of the ventral gray column can be subdivided further into motonuclear columns representative of muscle groups and peripheral nerves present in the limbs. GSE neurons that innervate proximal limb muscles are located in the ventral portion of the lateral part of the ventral gray column. Those innervating the more distal limb muscles are in the dorsal portion. These motonuclear columns have been identified by transecting peripheral nerves or ablating specific muscles and then observing in the spinal cord ventral gray column the retrograde chromatolysis of the neuronal cell bodies whose axons were destroyed in the experimental procedure.
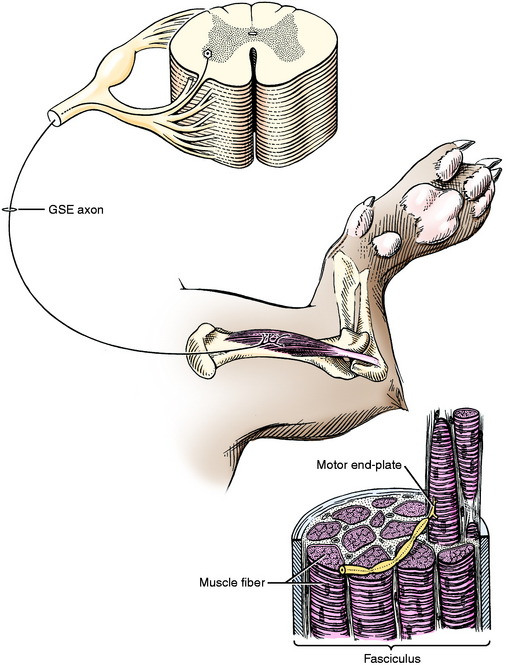
The dendritic zone of the multipolar GSE neuron is confined to the gray matter of the spinal cord adjacent to the cell body of that neuron. The axon courses through the white matter between the lateral and ventral funiculi to leave the spinal cord as part of a ventral rootlet. It continues in a ventral root and then into the spinal nerve, which that root forms. Then it travels into the limbs as part of a specific peripheral nerve that is distributed to a specific group of striated skeletal muscles (Fig. 5-2). The part of the axon that is located within the spinal cord (intramedullary) is myelinated by oligodendroglial cells, whereas the part of the axon located within the PNS is myelinated by Schwann cells.
At the level of the striated skeletal muscle, each axon of a GSE neuron divides into several branches. Each of these axonal branches ends on a muscle cell at a motor end-plate. Each adult muscle cell is innervated only by the axon of one motor neuron. In the fetus and newborn, more than one neuron may innervate a striated muscle cell, but during early postnatal development this polyneuronal innervation is reduced to a single neuron.82 The number of muscle cells innervated by one GSE neuron is called the motor unit. It varies from 100 to 150 muscle cells in the proximal limb muscles to 3 to 4 in extraocular muscles. Muscles involved in functions that require a large degree of coordination are innervated by motor neurons with small motor units (only a few muscle cells per neuron).
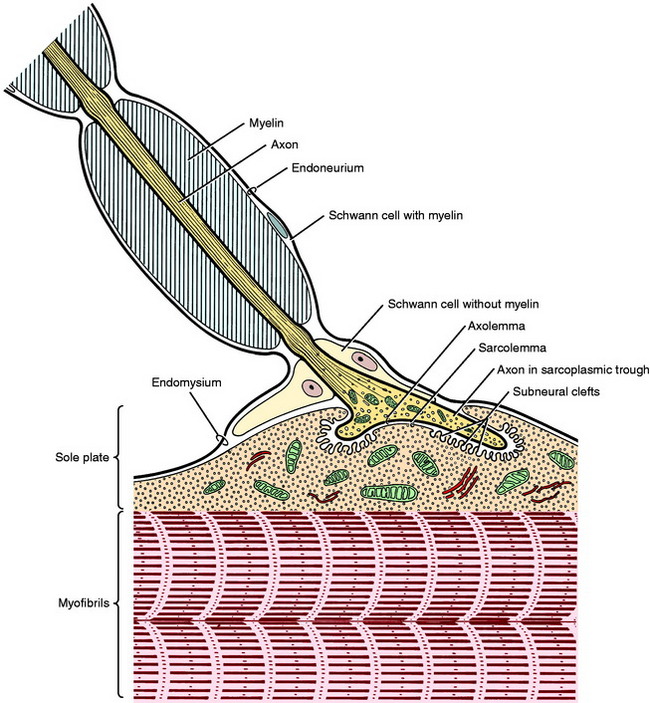
The strength of a muscle contraction depends on the number of motor units activated in a muscle. At the motor end-plate the myelin sheath is absent, and the axon terminates in several small branches that form a cluster in a localized area near the longitudinal center of the muscle cell. Each of these small branches terminates on specialized modification of the sarcolemma. This terminal is called the neuromuscular ending, junction, or synapse (Fig. 5-3).
When the action potential traveling along the axon arrives at the neuronal presynaptic membrane, calcium channels on the axolemmal surface open. The increase in axoplasmal calcium triggers the release of acetylcholine from the synaptic vesicles into the synaptic cleft where the acetylcholine molecules bind to the receptors in the sarcolemma of the sole plate. This binding opens these sodium channels and the influx of sodium results in depolarization of the sole plate region; if enough channels are opened, the depolarization spreads, resulting in muscle cell contraction. The acetylcholine released into the synaptic cleft is rapidly eliminated by diffusion away from the cleft site and by hydrolysis by acetylcholinesterase that is released into the cleft through the muscle cell membrane.
SPINAL CORD SEGMENTS: VERTEBRAL COLUMN
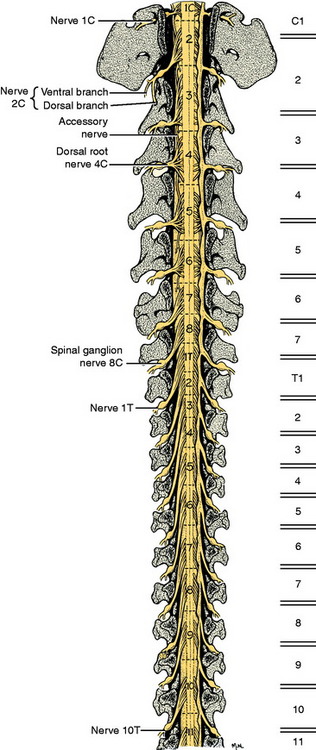
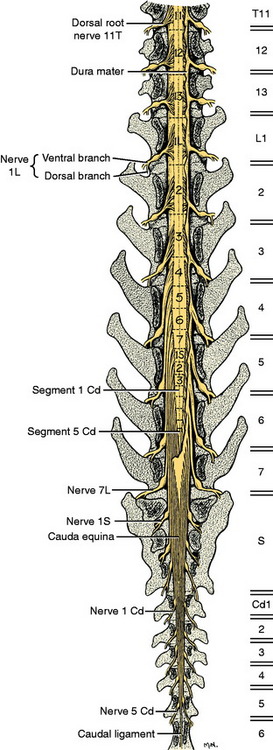
For clinical purposes, it is important to know in which spinal cord segments the neuronal cell bodies of the GSE motor neurons are located, whose axons are in specific peripheral nerves, and to know the specific vertebrae (vertebral foramen) in which these segments are found. For example, if your neurologic examination of a medium-sized dog determines that your patient has a deficiency localized to the sciatic nerve innervation of the pelvic limb, the anatomic diagnosis includes all components of that nerve as well as the neuronal cell bodies in the L6, L7, and S1 spinal cord segments. These spinal cord segments are located in the L4 and L5 vertebral foramina and their spinal nerves course caudally in the vertebral canal to leave through the intervertebral foramina between vertebrae L6 and L7, L7 and S1, and S1 and S2.57 In the dog, the spinal cord is composed of about 36 segments: 8 cervical, 13 thoracic, 7 lumbar, 3 sacral, and usually 5 caudal. Each segment has a number of dorsal and ventral rootlets that attach respectively to the dorsolateral and ventrolateral aspects of each side. The segmental spinal ganglion is found in the dorsal root just prior to its union with the ventral root at the level of the intervertebral foramen. The spinal nerve emerges from the intervertebral foramen and immediately branches into a dorsal and a ventral branch. Thus each spinal cord segment is connected to the tissues of the body by a spinal nerve on each side. The dorsal branch innervates epaxial tissues and the ventral branch innervates hypaxial tissues, which include the limbs.
The development in the embryo of the spinal cord segments and that of the vertebral column are closely related, which accounts for the manner in which the roots and spinal nerves of each segment are distributed among the vertebrae. The first cervical spinal nerves leave the vertebral canal through the lateral vertebral foramina in the arch of the atlas (Fig. 5-4). All the remaining spinal nerves leave this canal between vertebrae via intervertebral foramina or though the sacral foramina. This is also true for the cat. Other species have a variable number of vertebrae with lateral vertebral foramina. The spinal nerves of the second through the seventh cervical spinal cord segments leave the canal through the intervertebral foramina cranial to the vertebra of the same number. Therefore, the seventh cervical spinal nerves leave the vertebral canal cranial to the C7 vertebrae (see Fig. 5-4). Because there are always only seven cervical vertebrae and there are eight cervical spinal cord segments, the spinal nerves of the eighth cervical spinal cord segment leave the vertebral canal cranial to the first thoracic vertebra. All the remaining spinal nerves leave the vertebral canal through the intervertebral foramina that are caudal to the vertebrae of the same number. This segmental relationship of the spinal cord and vertebral column is established in the embryo.
After birth, as the animal grows, there is more growth in the vertebral column than in the spinal cord, and this alters the relationship of the spinal cord segments to the vertebrae by means of a cranial displacement of most of the spinal cord segments. In the dog, only the first and second cervical spinal cord segments and the last two thoracic and first two or three lumbar segments lie in the vertebral canal within the vertebra of the same numbers. All the remaining segments reside in the canal cranial to the vertebrae of the same number. To accommodate for this cranial displacement, the spinal nerves must grow in length because the place where they exit the vertebral canal can not be altered. This is especially evident in the caudal lumbar and sacral vertebrae, where the arrangement of the long spinal roots and nerves adjacent and caudal to the conus medullaris is called the cauda equina for its resemblance to the tail of a horse. (See the table and discussion in Chapter 4, where the positions of the sacral segments within the vertebral canal are described.) This cranial displacement of the caudal lumbar, sacral, and caudal segments is most apparent in the large dog breeds in which the three sacral segments are located in the fourth lumbar vertebra. In all dogs and cats, the only nervous system tissue in the vertebral foramen of L7 is the collection of spinal nerves passing caudally to their respective intervertebral foramina to leave the vertebral canal (Fig. 5-5).
FUNCTION
The GSE portion of the LMN provides the final motor innervation of the muscles whose contractions are necessary to maintain posture, support weight, and provide the gait. They are also the motor component of the spinal reflexes that are tested in the neurologic examination. Knowledge of the anatomy of these reflexes is critical to localizing lesions to portions of the PNS or the spinal cord.
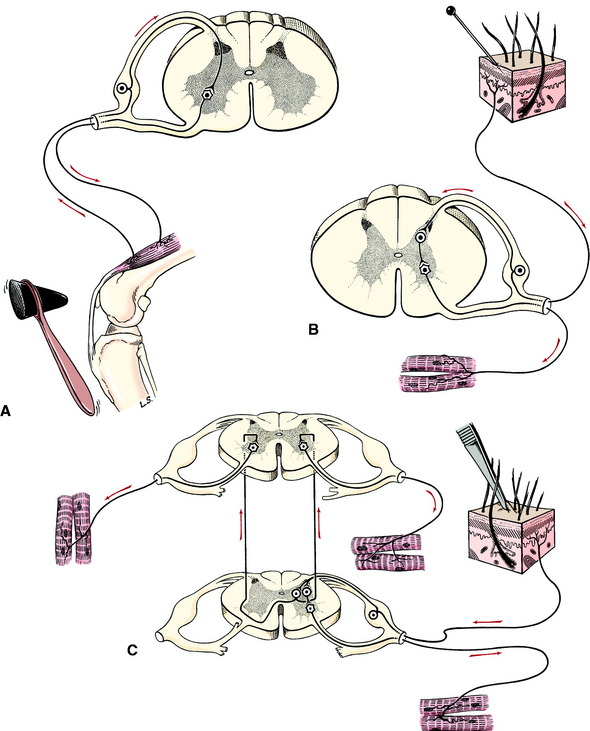
The sensory components of these reflexes are neurons of the general somatic afferent (GSA) and general proprioceptive (GP) systems. These systems are described in Chapter 9. These sensory neurons consist of a dendritic zone (receptor) in the skin or neuromuscular spindle and an axon that courses through a specific peripheral nerve, spinal nerve, and dorsal root and enters the spinal cord dorsal gray column of the corresponding spinal cord segment. Here it usually terminates in a telodendron on a dendritic zone of a second neuron, whose cell body is located in the dorsal gray column (Fig. 5-6). The cell body of the GSA or GP neuron initially stimulated is located in the spinal ganglion at the lateral aspect of the dorsal root.
The patellar reflex (see Fig. 5-6) is a tendon reflex that is composed of only two neurons. The sensory neuron terminates directly on the GSE neuron in the ventral gray column without involving a synapse on a second neuron in the gray column. The peripheral sensory neuron of the flexor, or withdrawal spinal reflex, has its telodendron on an interneuron in the gray matter that in turn terminates on a GSE neuron in the ventral gray column.
For these spinal reflexes to function, they need only the peripheral nerve components and their associated spinal cord segments. They will still function when these segments have been cut off and isolated from the rest of the CNS. For example, if you examined a dog 2 days after it had been hit by a car, which fractured the thirteenth thoracic vertebra and transected the thirteenth spinal cord segment, the spinal reflexes in the pelvic limbs and perineal region would still function because there was no direct disturbance to the spinal cord segments and peripheral nerves involved with these reflexes. In order to interpret these spinal reflexes properly it is vital to understand their anatomic components (Table 5-1). This is critical to making an accurate anatomic diagnosis of peripheral nerve and spinal cord disorders.
PELVIC LIMB AND PERINEAL REFLEXES
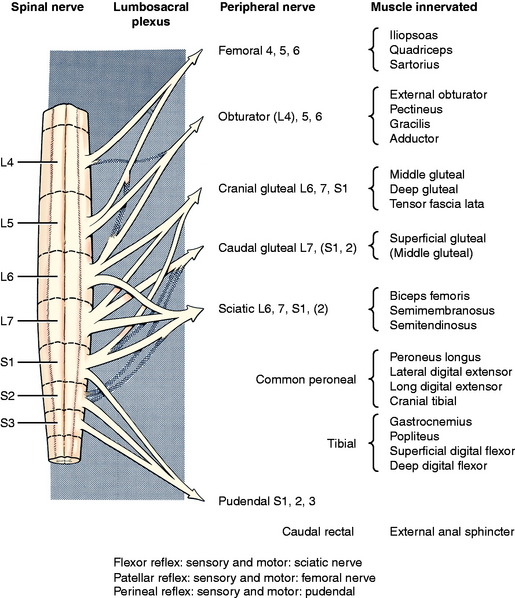
The patellar tendon reflex is the only reliable tendon reflex and the only one that I (Alexander de Lahunta) test in my examinations. Both the sensory and motor components are in the femoral nerve. The femoral nerve is formed from the spinal nerves of the L4, L5, and L6 spinal cord segments (Fig. 5-7). The L5 segment makes the largest contribution to this nerve.141 The L6 segment may not contribute to this nerve in some dogs. The patient should be held in lateral recumbency and must be relaxed. This reflex cannot be tested in a struggling patient. Lightly strike the patellar tendon with a blunt instrument. A pediatric patellar hammer or pleximeter is the most satisfactory instrument for veterinarians, but the handle of a pair of scissors will work as well. In my experience (Eric Glass) the pediatric pleximeter is especially useful in cats and small dogs, but a larger pleximeter or heavy bandage scissors may be more reliable in large dogs such as German shepherds. This will elicit a brief extension of the stifle if all components of the femoral nerve are functioning. We usually grade the response as normal (+2), depressed (+1), or brisk (+3). The response is called clonus if, with one stimulation, repetitive stifle extensions occur. The paw will appear to tremor. There are degrees of clonus but we do not find them to be of much help in prognosis. Occasionally, this patellar reflex will not be present in the nonrecumbent limb but will be present when the dog is rolled over and you test the reflex when that limb is on the recumbent side. We do not know the reason for this but have learned never to record that the reflex is absent until it has been tested in both positions. Be aware that this reflex may be depressed or even absent in some old dogs that have no other recognizable neurologic abnormality.87
The flexor or withdrawal reflex in the pelvic limb is a test primarily for the sciatic nerve and its spinal cord segments L6, L7, and S1. Within the sciatic nerve, the neurons that will be associated with the peroneal nerve tend to be components of the L6 and L7 spinal cord segments, and those associated with the tibial nerve are components of the L7 and S1 segments. The S2 components innervate primarily muscles in the pelvis that do not participate in this test or in the animal’s posture or gait. The sensory component of the reflex depends on the area of skin that is stimulated. In a routine exam, the skin at the base of the claw of the fifth digit is compressed using a pair of forceps. Finger pressure can be used but is not always sufficient, in our experience. This area of skin is innervated by cutaneous branches of the peroneal nerve dorsally and by the tibial nerve on the plantar surface. The motor response is a flexion of all the joints in the limb to withdraw the limb from the stimulus. Except for the hip, flexion of the pelvic limb is a function of the GSE components of the sciatic nerve. The major flexor muscle of the hip is the iliopsoas, which is innervated by all the lumbar spinal nerve ventral branches, with a contribution caudally from the femoral nerve. The latter also innervates the rectus femoris, which is the one component of the quadriceps muscle that flexes the hip. Because of this anatomy, a patient with complete sciatic nerve dysfunction will have no reflex if the fifth digit is stimulated, but if the first digit is stimulated, the hip will flex to pull the limb away from the stimulus but the rest of the joints will not flex. The first digit usually receives its cutaneous innervation from the saphenous nerve branch of the femoral nerve. Be sure to look for this disparity. The same strong hip flexion in the absence of any flexion in the other joints will occur with severe but not complete sciatic nerve dysfunction when the fifth digit is stimulated. As a rule, there is more clinical evidence of loss of the motor function with some preservation of the sensory function in a partially compromised peripheral nerve.
The flexor reflex is not only a test of the reflex arc that utilizes the sciatic nerve; it is also a test for nociception, which requires the sensory portion of the peripheral nerves involved with the reflex plus a pathway through the spinal cord and brainstem to the neocortex. This is described with the GSA system in Chapter 9. Because of this nociceptive pathway, you must be gentle with the degree of compression of the digit that you use or you may rightfully be abused by your patient.
THORACIC LIMB REFLEXES
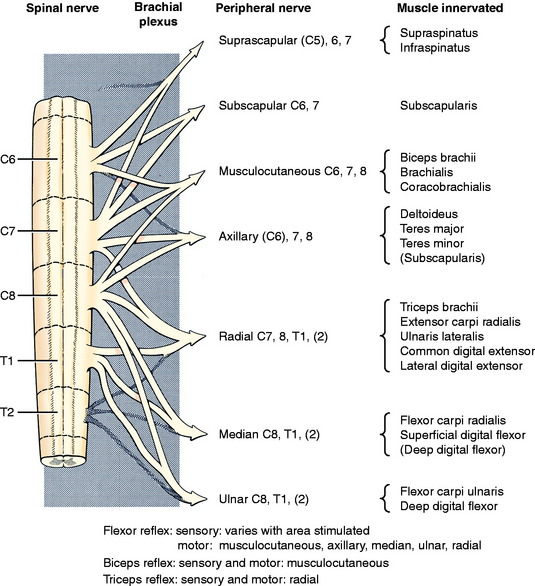
As a rule, we rely only on the flexor-withdrawal reflex in the thoracic limb. In reality, it is a crude test of many anatomic components. The peripheral sensory component depends on the area of skin that is stimulated by compression. These areas are more accurately defined in Chapter 9, the GSA system. Compression of the lateral digit stimulates primarily the cutaneous endings of branches from the ulnar nerve dorsally and from the ulnar and median nerves on the palmar surface. The muscles that contract to flex the joints and withdraw the limb are innervated by the GSE-LMN systems present in many nerves: for shoulder flexion, it is the axillary, radial, and thoracodorsal nerves; for elbow flexion, it is the musculocutaneous nerve; for carpal and digital flexion, it is the median and ulnar nerves.
These nerves have their central components in the cervical intumescence, which includes the spinal cord segments (C5), C6, C7, C8, T1, and T2. The ventral branches of the spinal nerves from these segments intertwine medial to the shoulder to form the brachial plexus from which the specific named peripheral nerves emerge (Fig. 5-8). For example, the radial nerve contains primarily branches from spinal nerves and segments C7, C8, and T1. All of these components must be intact for the withdrawal reflex to be normal. Remember that, as in the pelvic limb, the stimulus used is a noxious stimulus that tests the nociceptive pathway as well as the flexor reflex. Always start with a gentle compression.
There are three tendon-muscle reflexes that are used by some neurologists, but the reliability of these reflexes may be questioned. For the biceps reflex, hold the elbow in partial extension with your first finger on the distal bellies of the biceps brachii and brachialis muscles. Lightly tap your finger with your patellar hammer or some other blunt instrument and observe a slight flexion of the elbow or feel the muscles contract beneath your finger. This is a test for the musculocutaneous nerve and the C6 and C7 spinal cord segments. For the triceps reflex, hold the elbow relaxed in slight flexion and lightly tap the triceps tendon just proximal to the olecranon tuber and observe for a slight elbow extension. This tests for the radial nerve and spinal cord segments C7, C8, and T1. For the extensor carpi radialis reflex, hold the thoracic limb so that the carpus is relaxed. Lightly tap the proximal belly of this muscle and observe for carpal extension. This tests the extension of the radial nerve into the antebrachium by its deep branch. The presence of these tendon-muscle reflexes indicates that the nervous system components involved are able to function. Their absence may be of no consequence because these reflexes are not always present in all normal animals.
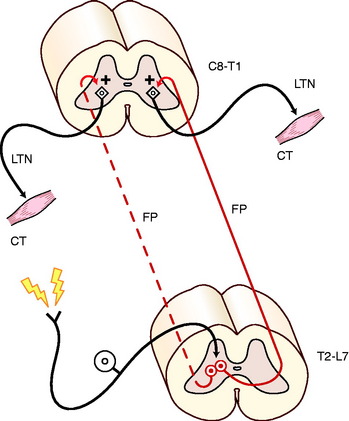
The cutaneous trunci reflex involves a larger portion of the spinal cord (Fig. 5-9). It is useful for locating C8 and T1 spinal cord lesions and their contributions to the lateral thoracic nerve, and it is also useful for locating the level of a transverse thoracolumbar spinal cord lesion. The sensory stimulus is a mild compression of the skin of the trunk using forceps, and the motor response is a brief contraction of the cutaneous trunci muscle, which causes the skin of the trunk to twitch. This reflex is usually performed using progressive stimuli starting in the caudal lumbar region and progressing cranially along the dorsal midline of the trunk. The cutaneous nerves that are stimulated are from the dorsal branches of the lumbar and thoracic spinal nerves (see Fig. 5-9). The sensory impulse courses through the dorsal roots and enters the dorsal gray column of each respective spinal cord segment. Here the axons of the GSA neurons synapse on long interneurons whose axons enter the fasciculus proprius on both sides of the spinal cord but predominantly the contralateral side. The fasciculus proprius is the white matter of the lateral funiculus that is adjacent to the gray matter. These axons course cranially to the T1 and C8 spinal cord segments where they terminate in the ventral gray column by synapsing on GSE neurons whose axons emerge from the brachial plexus as a component of the lateral thoracic nerve that innervates the cutaneous trunci muscle. Any disorder that interrupts this pathway will interfere with this reflex. On rare occasions this reflex cannot be elicited in a normal dog. Sometimes it takes rigorous stimulation to elicit it. Normal dogs vary in how far caudally in the lumbar region the reflex can be stimulated. Most will show the reflex by the middle of the lumbar region. Its use in locating the level of a transverse spinal cord lesion is described with spinal cord disorders.
LOWER MOTOR NEURON DISEASE: NEUROMUSCULAR DISEASE
Clinical Signs
The dictionary definition of paresis is weakness. The student tends to interpret this to mean loss of muscle strength with loss of tone and difficulty supporting weight, which characterizes the paresis caused by LMN disease. However, in medicine there are two qualities of paresis. When severe, both forms can result in complete paralysis and inability to move, but the quality of the paralysis is very different in the two forms. These two forms are LMN and upper motor neuron (UMN) paresis or paralysis. The word paralysis should be used only when there is complete absence of any voluntary movement. LMN paresis is described in this chapter. UMN paresis is described with the anatomy of that system in Chapter 8. The two forms are compared in that chapter.
Neurologists define paresis as a deficiency in the generation of the gait or in the ability to support weight. The lack of ability to support weight characterizes LMN paresis. If the patient is ambulatory, the gait will be short-strided and will appear as a lameness. The gait in LMN disease is identical to that of an animal that has discomfort when the diseased limb attempts to support weight. The inability to support weight looks the same as when an animal expresses pain whenever weight is supported. This animal walks as you would with a stone in your shoe. The stride is shortened. It is important to rule out any orthopedic disorder in your evaluation of an animal with LMN disease by doing a complete orthopedic examination. When you see a shortened stride in the gait, always ask yourself if it is because the patient is unable to support its weight or is unwilling to support its weight. If multiple limbs are affected with LMN disease, the animal will tend to collapse and have difficulty standing up from a recumbent position. When both pelvic limbs are affected, the animal may walk by simultaneously flexing both hips to advance the limbs. This is referred to as bunny-hopping. Be aware that animals that have pelvic or pelvic limb noxious stimuli may also bunny-hop as an expression of pain. When standing, the affected limbs may exhibit a tremor in the muscles, and the affected animal may continually shift its weight from one limb to another and look for an opportunity to lie down. This is especially evident in horses with diffuse LMN disease. An animal with diffuse LMN disease that is still ambulatory may stumble on uneven surfaces. This state is often confused with ataxia, which implies a sensory system abnormality—a general proprioceptive, vestibular, or cerebellar disorder. LMN disease does not cause ataxia, only paresis!
Severe diffuse LMN disease produces the same degree of paralysis as a severe focal midcervical spinal cord lesion. Recumbent immobile patients appear to be the same as each other. The first clue that they are very different will be when you attempt to pick the patient off the ground surface. The patient with diffuse LMN disease will be atonic and just hang limply over your forearms. When you lift the patient up and down, the limbs will flop uselessly like wet dish rags. This is a flaccid (originally pronounced flack-sid) paralysis. When all four limbs are affected, it is referred to as a tetraplegia or quadriplegia. Thus a patient with severe diffuse LMN disease has flaccid tetraplegia. A patient with a severe focal cervical spinal cord lesion will be paralyzed in recumbency because of the loss of function in its UMN pathways, resulting in the inability to generate any LMN function and releasing the reflex arcs from inhibition. The latter results in hypertonia that is referred to as spasticity. This will be obvious when you attempt to pick up the recumbent animal. All the muscles will feel taut, giving the trunk and limbs a remarkably stiff feeling. This will be especially obvious as you lift the patient up and down or try to flex any of the limbs. This is spastic paralysis of all four limbs, or spastic tetraplegia. Video 10-24 shows two dogs (described in Chapter 10) that make it easy to appreciate the comparison.
The patient with the severe diffuse LMN disease described earlier will have no tone in the limbs when they are manipulated—atonia—and no patellar tendon or flexor reflexes—areflexia. The latter reflex requires a noxious stimulus, and many diffuse LMN diseases do not affect the GSA pathways; therefore, nociception may be intact. The only evidence of nociception will be jaw and facial movements and an increase in the rate of respirations because any voice sounds may be absent or muffled. Animals that are recumbent because of LMN disease but still have some voluntary movements may exhibit hypotonia and depressed spinal reflexes. In the diffuse LMN disease known as myasthenia gravis, the recumbent animal may still have normal tone and normal spinal reflexes. The patient that has diffuse LMN disease and is still able to ambulate may have reduced muscle tone and reflexes or they may be normal. Remember to examine the muscle tone and reflexes in the anus, perineum, and tail because they will be affected with sacrocaudal LMN disorders.
Wallerian Degeneration
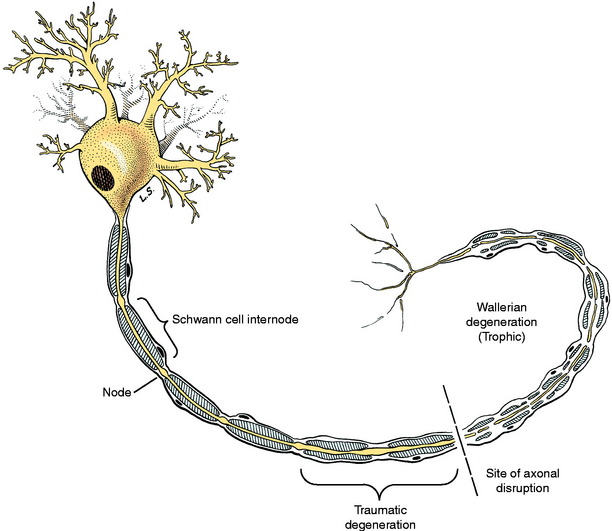
Wallerian degeneration is a trophic degeneration that occurs in the neuron at the site of the lesion and travels in a distal direction from the cell body as follows (Fig. 5-10). The axon degenerates through a process of swelling and subsequent granulation that takes about 3 to 4 days. The myelin degenerates simultaneously with the axons. There is a close interaction between the axon and its myelin, and myelin cannot survive if the axon degenerates. In this process of wallerian degeneration, there is a secondary demyelination that includes the formation of swellings along the internodes, called ellipsoids, and the fragmentation of myelin into droplets. Digestion chamber is a term used for a myelin ellipsoid containing axonal granules. The telodendron and motor end-plate disintegrate. The Schwann cells that are now reduced to their nuclei and cytoplasmic organelles rapidly proliferate to form a column of cells known as a Büngner band. The adjacent endoneurial cells also proliferate. These columns of Schwann cells provide pathways for regenerating axons to follow to the target that was denervated. They also provide growth factors that induce the outgrowth of axonal buds from the proximal portion of the neuron where the axon is still intact. This regeneration begins in about 7 days. Each axon puts out a number of processes called axonal buds that grow into the existing cords of proliferating Schwann cells. The rate of growth is about 1 to 4 mm per day. This process is dependent on close proximity of the axonal buds and the Büngner bands. With this knowledge, you can estimate for the owner of a patient that has a peripheral nerve injury how long it will take for some evidence of regeneration to occur by measuring the distance from the site of the lesion to the middle of the denervated muscles. Using the slowest rate (1 mm/day), the distance in millimeters is the same as the number of days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree