Chapter 6 Lower Motor Neuron
General Somatic Efferent, Cranial Nerve
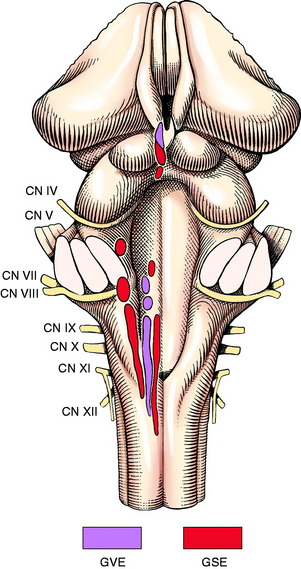
The general somatic efferent (GSE) lower motor neurons (LMN) in cranial nerves innervate the voluntary striated skeletal muscle derived from occipital somites (i.e., tongue: cranial nerve XII) and from the head somitomeres that migrate into the branchial arches (i.e., extraocular muscles: cranial nerves III, IV, and VI; muscles of mastication: cranial nerve V; facial muscles: cranial nerve VII; larynx: cranial nerve X; and pharynx and esophagus: cranial nerves IX and X). Like spinal nerves, these cranial nerves contain neurons relating to other functional systems. Cranial nerves III, VII, IX, and X contain general visceral efferent (GVE) preganglionic neurons, and most contain some form of sensory neurons. The cell bodies of these GSE neurons are found in nuclei in the brainstem from the mesencephalon through the caudal medulla (Fig. 6-1).
CRANIAL NERVE III: OCULOMOTOR NEURONS
Anatomy
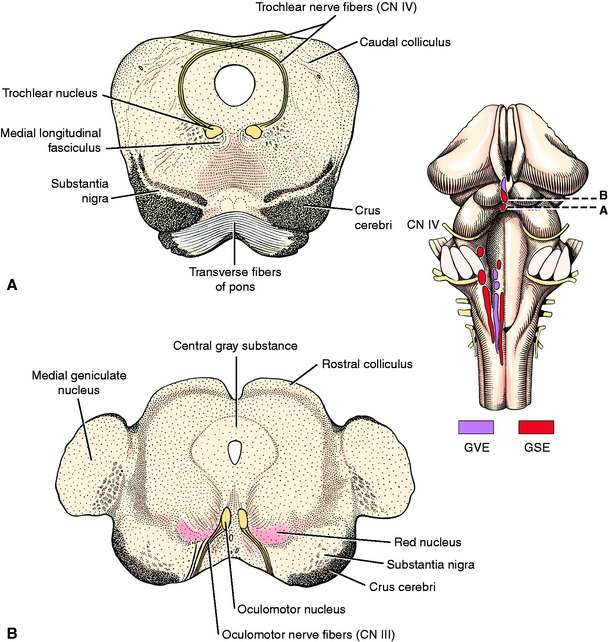
The GSE neuronal cell bodies of oculomotor neurons are located in the oculomotor nucleus in the rostral mesencephalon at the level of the rostral colliculi (Fig. 6-2; see also Figs. 2-6, 2-7, and 6-1). The nucleus is adjacent to the midline within the ventral portion of the central gray substance that surrounds the mesencephalic aqueduct. This nucleus is rostral to the trochlear nucleus and caudal to the pretectal nucleus. The GSE neuronal cell bodies are in the more caudal portion of the oculomotor nucleus, and the GVE parasympathetic preganglionic neuronal cell bodies are in the more rostral portion of this nucleus. These GVE neurons are described in Chapter 7. The oculomotor nucleus is dorsal and medial to the red nucleus. The medial longitudinal fasciculus is located just medial and ventral to this oculomotor nucleus. One function of this fasciculus is to interconnect the oculomotor, trochlear, and abducent neurons that innervate the extraocular muscles to permit coordinated conjugate movements of the eyes.
The axons of the GSE oculomotor neuronal cell bodies pass ventrally through the reticular formation of the mesencephalic tegmentum medial to the red nucleus, substantia nigra, and crus cerebri. They emerge on the lateral side of the interpeduncular fossa to form the oculomotor nerve on the medial side of the crus cerebri. This nerve courses rostrally in the middle cranial fossa beside the pituitary gland, adjacent to but not in the cavernous sinus. The oculomotor nerve leaves the cranial cavity through the orbital fissure and within the periorbita; the oculomotor nerve branches to supply the medial, dorsal, and ventral recti muscles, the ventral oblique muscle, and the levator palpebrae superioris muscle.
CRANIAL NERVE IV: TROCHLEAR NEURONS
Anatomy
The GSE neuronal cell bodies are located in a very small nucleus in the caudal mesencephalon at the level of the caudal colliculi (see Fig. 2-8 and Figs. 6-1 and 6-2). This trochlear nucleus is adjacent to the midline in the ventral portion of the central gray substance that surrounds the mesencephalic aqueduct. It is caudal to the larger oculomotor nucleus. The medial longitudinal fasciculus, which connects the vestibular nuclei with these nuclei that innervate the extraocular muscles, lies just medial and ventral to the trochlear nucleus.
The axons of the GSE neuronal cell bodies in these trochlear nuclei course progressively in lateral, dorsal, and caudal directions to enter the rostral medullary velum, where they continue across this velum to the opposite side and emerge just caudal to the caudal colliculus (see Fig. 2-9). These axons continue rostrally and ventrally on the side of the mesencephalon to reach the floor of the middle cranial fossa. They continue rostrally beside the pituitary gland adjacent to but not in the cavernous sinus and leave the cranial cavity through the orbital fissure. Within the periorbita, the trochlear neurons innervate the dorsal oblique muscle. This is the only cranial nerve with GSE neurons that innervates a muscle solely on the side opposite from its nucleus (see the discussion of the clinical signs caused by lesions that affect these trochlear neurons; it follows the description of the anatomy of the abducent neurons).
CRANIAL NERVE VI: ABDUCENT NEURONS
Anatomy
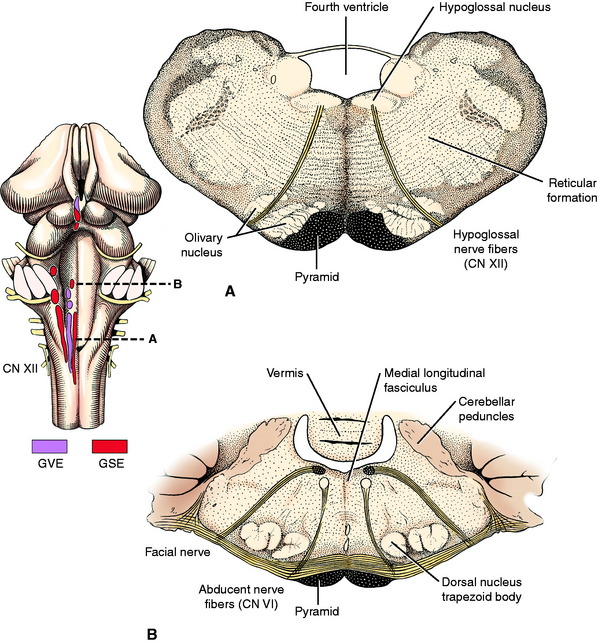
The GSE neurons of the abducent nerve are located in the abducent nucleus in the rostral medulla (see Fig. 2-12). This is a small nucleus at the level of the medulla where the caudal cerebellar peduncle merges with the cerebellum. It is adjacent to the midline and floor of the fourth ventricle. The GSE axons of the genu of the facial nerve pass over this nucleus (Fig. 6-3; see also Fig. 6-1 and 6-24). The axons of the abducent neuronal cell bodies in the nucleus pass directly ventrally through the reticular formation of the medulla medial to the distinct dorsal nucleus of the trapezoid body. They pass through the trapezoid body and emerge just lateral to the pyramid. The abducent nerve courses rostrally on the floor of the middle fossa beside the pituitary gland, adjacent to but not in the cavernous sinus, and leaves the cranial cavity through the orbital fissure. It branches within the periorbita to innervate the lateral rectus and retractor bulbi muscles.
FUNCTION OF CRANIAL NERVES III, IV, AND VI
Function
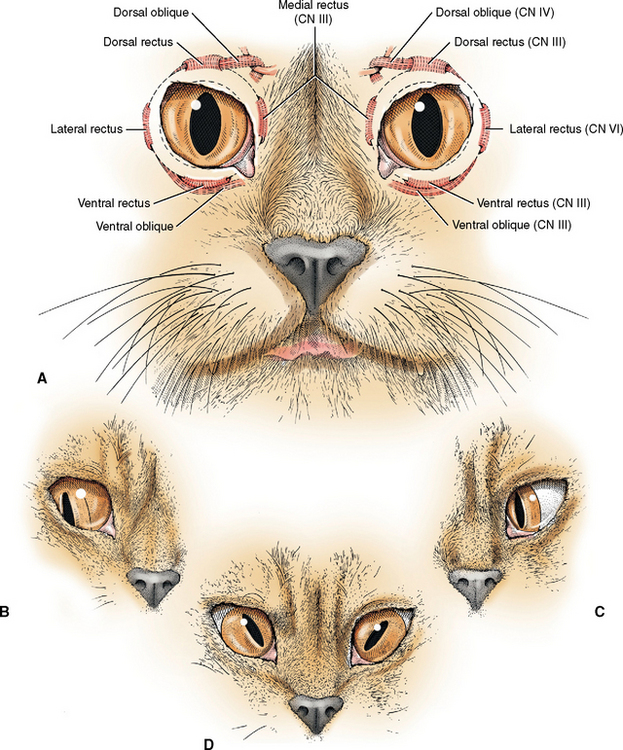
To understand the clinical signs resulting from lesions that interfere with the function of one or more of these three cranial nerves that innervate the extraocular muscles, the normal action of these muscles on the eyeball must be understood. If the eyeball is assumed to have three axes for rotation, the muscles can be grouped into three opposing pairs. If you assume a horizontal axis through the center of the eyeball, the dorsal rectus will elevate the eyeball, and the ventral rectus will depress the eyeball. If you assume a vertical axis through the center of the eyeball, the medial rectus will adduct the eyeball and the lateral rectus will abduct it. If you assume an anterior-to-posterior axis through the center of the eyeball, the dorsal oblique will intort the eyeball, or rotate the dorsal portion medioventrally toward the nose, and the ventral oblique will extort the eyeball, or rotate the dorsal portion lateroventrally away from the nose. These muscles do not function alone but continuously act together in a synergistic or antagonistic manner to provide for conjugate movements of both eyeballs in the same direction at the same time. This is easiest to appreciate by considering the action of the medial and lateral recti muscles in horizontal conjugate movements. To test the action of these muscles as part of your cranial nerve examination, you obviously cannot depend on your patient to respond to your command to look in different directions. However, the function of the oculomotor nerve to the medial rectus and the abducent nerve to the lateral rectus is readily assessed by testing for normal or physiologic nystagmus, a vestibuloocular test.
Nystagmus is defined as involuntary movements of the eyes, and there are normal and abnormal forms of nystagmus. The normal nystagmus that occurs with this test is a jerk nystagmus, meaning that there is a fast and a slow phase of the eye movement. The direction of the fast phase defines the direction of the nystagmus. Stand over your small animal patient so that you both are headed in the same direction; with large animals, stand so that you face them and can observe both eyes. Move the head at a moderate rate from one side to the other and keep repeating this as you watch the movements of the eyes. It may help in small animals to hold the eyelids open as you do this test so that you can observe the limbus. Moving the head stimulates the sensory receptors in the inner ear that are innervated by the vestibular portion of cranial nerve VIII. Impulses are relayed into the vestibular nuclei in the medulla and then passed rostrally via the medial longitudinal fasciculus to the abducent, trochlear, and oculomotor nuclei. As you move the head to the right, both eyes will exhibit a rapid movement, a jerk, toward the right. This is a normal right nystagmus. As you move the head back to the left, both eyes will jerk to the left, a normal left nystagmus. In neuroanatomic terms, when the head is moved to the right, the right eye will jerk to the right via contraction of the lateral rectus innervated by the abducent nerve, and at the same time the left eye will jerk to the right via the contraction of the medial rectus innervated by the oculomotor nerve. When you swing the head back to the left, the opposite innervations and muscles will be activated. This action tests the function of cranial nerves III and VI as well as the vestibular nerve (VIII) and the pathway of the medial longitudinal fasciculus through the brainstem. These are conjugate eye movements related to the movement of the head and have nothing to do with vision. This test can be performed in blind animals.
Clinical Signs
Lesions of the abducent neurons cause paralysis of the lateral rectus and retractor bulbi muscles. Loss of the lateral rectus innervation results in a medial strabismus (Fig. 6-4) due to the unopposed contraction of the medial rectus muscle. When you test for normal nystagmus by moving the head from one side to the other in a horizontal plane, the affected eye will not abduct when the head is moved toward that eye. Carefully compare the degree of abduction of the two eyes to appreciate this clinical sign. There is no reliable test for eyeball retraction by the retractor bulbi muscle because all the rectus muscles also have this retractor function.
Lesions of the trochlear neurons cause paralysis of the dorsal oblique muscle, and you will observe a strabismus caused by the unopposed contraction of the ventral oblique muscle. This strabismus will be contralateral to a nuclear lesion in the mesencephalon and ipsilateral to a trochlear nerve lesion after it emerges from the rostral medullary velum. In cats, which have vertical pupils, the dorsal, 12-o’clock position of the pupil will be extorted, deviated laterally. In horses and ruminants, which have horizontal pupils, the medial aspect of the pupil will be extorted dorsally. This is often referred to as a dorsomedial strabismus. In ruminants, this is a clinical sign that commonly occurs in severe cases of a metabolic disorder caused by thiamin deficiency and is referred to as polioencephalomalacia. This is thought to represent dysfunction of the trochlear nucleus. In dogs, with their round pupils, extorsion cannot be appreciated unless you do a fundic examination and look for the position of the superior retinal vein that normally courses in a superior direction from the optic disk. It will be deviated laterally in a trochlear nerve paralysis. Trochlear nerve strabismus is rarely observed except in the ruminant disorder just described.
Lesions of the GSE oculomotor neurons cause paralysis of the dorsal and ventral rectus, the ventral oblique, and the medial rectus muscles as well as the levator palpebrae superioris. Loss of the function of the levator muscle causes a smaller palpebral fissure due to ptosis resulting from the inability to elevate the upper eyelid. Paralysis of the extraocular muscles causes a ventrolateral strabismus (see Fig. 6-4). The lateral deviation is explained by the unopposed contraction of the lateral rectus muscle. The mild ventral deviation has no obvious explanation other than the collective result of the loss of function of the other extraocular muscles innervated by the oculomotor nerve. The loss of the ability to adduct the eye can be readily observed when you test the patient for normal nystagmus by moving the head in a horizontal plane. The affected eye will not adduct fully when the head is moved in the direction of the normal eye. In small animals, when the head and neck are extended, the eyes normally move dorsally to stay in the center of the palpebral fissure. Paralysis of the dorsal rectus muscle prevents this in the affected eye, and you will observe the dorsal aspect of the sclera when you perform this movement. In the horse and ruminants, especially cattle, the eyes normally do not move dorsally as the head and neck are extended, and you normally observe the dorsal aspect of the sclera in both eyes.
Lesions that involve all components of the oculomotor nerve also cause dilated pupils that are unresponsive to light due to the loss of the GVE parasympathetic preganglionic neurons that innervate the iris constrictor muscle.36 This is described in Chapter 7 when we discuss the GVE system.
Strabismus may also occur with some congenital anomalies in the architecture of the central projections of the visual system. A mild bilateral medial strabismus is observed in Siamese cats. This breed has a higher portion of retinal ganglion cell neurons whose axons cross at the optic chiasm and project to the contralateral lateral geniculate nucleus.33
Diseases
The most common lesion to involve one or more of these cranial nerves that innervate the extraocular muscles is a neoplasm that is retrobulbar or extraparenchymal (extraaxial or extramedullary) in the middle cranial fossa. Meningiomas and germ cell neoplasms are the most common in the middle cranial fossa to affect these cranial nerves.35 In the literature, the clinical signs of neoplasms located here that affect cranial nerves III, IV, and VI and the ophthalmic and maxillary nerves from V have been referred to as the cavernous sinus syndrome. This term should be discarded, because these nerves have nothing to do with the cavernous sinus other than to course by it to leave the cranial cavity. Pituitary macroadenomas rarely affect these nerves.
CRANIAL NERVE V: TRIGEMINAL NEURONS
Anatomy
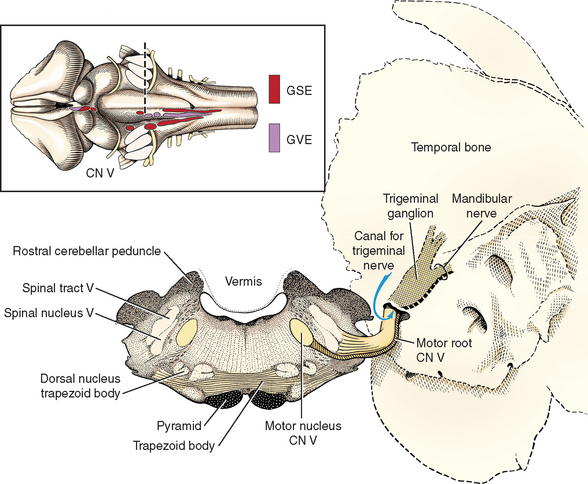
The GSE neuronal cell bodies are located in the motor nucleus of the trigeminal nerve, which is a small, well-defined nucleus in the pons (see Fig. 2-11). This nucleus is found at the level of the middle and rostral cerebellar peduncles just rostral to where all three cerebellar peduncles merge with the cerebellum. It is medial to the pontine sensory nucleus of the trigeminal nerve and dorsal to the dorsal nucleus of the trapezoid body (Fig. 6-5; see also Fig. 6-1).
Clinical Signs
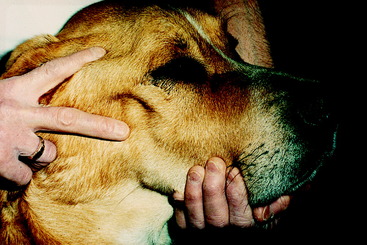
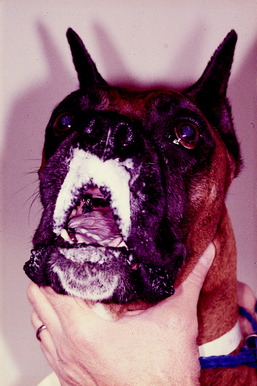
Unilateral loss of function of the trigeminal motor neurons will be recognized only when atrophy occurs in the muscles of mastication (Figs. 6-6 and 6-7). This will be observed more readily in the shorthaired breeds. In your neurologic examination, be sure to palpate these muscles or you will miss this important clinical sign, especially in longhaired breeds such as the old English sheepdog. In the horse, there may be a slight deviation of the lower jaw toward the normal side, but this may be difficult to recognize. There must be bilateral loss of function in these trigeminal motor neurons to result in failure to prehend food because the mouth cannot be closed. An open mouth due to a dropped jaw that the patient cannot close but that you can close manually requires the anatomic diagnosis of bilateral motor cranial nerve V.
Diseases
Malignant Nerve Sheath Neoplasm
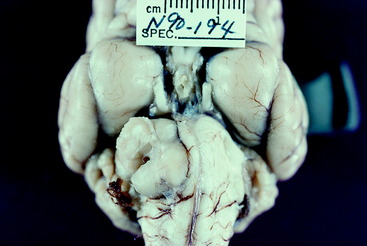
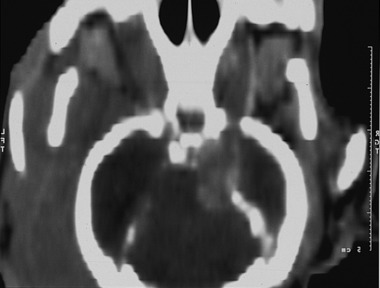
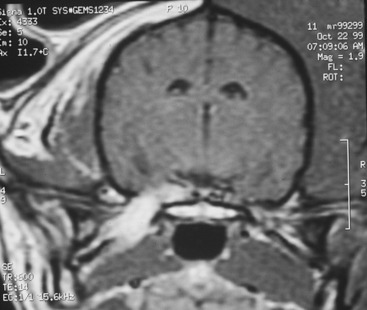
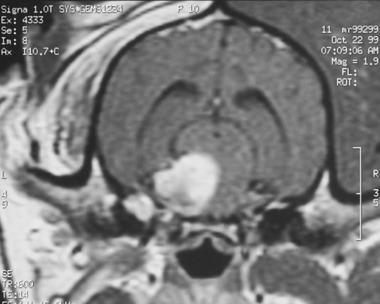
The most common lesion that causes a unilateral loss of the GSE-LMN in the trigeminal nerve is a malignant nerve sheath neoplasm. This is a neoplasm of Schwann cells that occurs in older dogs. The first indication of the neoplasm is usually recognition of atrophy in the muscles of mastication (see Figs. 6-6 and 6-7). There is no recognizable loss of jaw function. Be aware that the loss of general somatic afferent (GSA) neurons in the ophthalmic nerve from of the trigeminal nerve may cause a neurotrophic keratitis, which may be the first clinical sign observed. Often, there is no detectable loss of sensory function with this neoplasm, but it commonly expands into the cranial cavity and slowly compresses the brainstem, with its mass centered at the pons (Figs. 6-8 and 6-9). This will lead to other cranial nerve signs (facial and vestibulocochlear) on the same side and abnormal postural reactions and gait in the ipsilateral limbs. Computed tomography (CT) and magnetic resonance (MR) imaging will diagnose this mass lesion and determine the extent of its growth (Figs. 6-10 through 6-14). Surgical removal is difficult because of the location of the mass, but it has been described. Usually this neoplasm is treated by radiation, and corticosteroids are used to control perilesional edema. The trigeminal nerve is the most common cranial nerve to develop this nerve sheath neoplasm in animals.
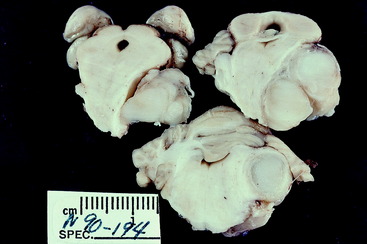
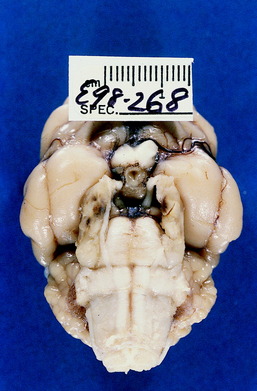
Figure 6-9 Transverse sections of the brain of the dog in Fig. 6-8, showing the severe compression of the mesencephalon and ventral metencephalon (pons). In addition to the atrophy of the right muscles of mastication, this dog exhibited right spastic hemiparesis and ataxia. However, the quality of the ataxia reflected predominantly dysfunction of the vestibular system rather than of the general proprioceptive system.
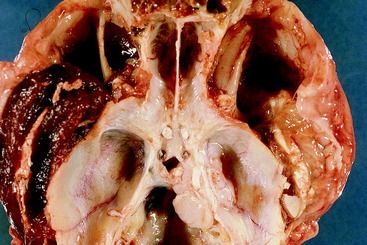
Figure 6-11 Dorsal view of the floor of the cranial cavity in the dog shown in Fig. 6-10. Note the large mass at the level of the canal for the trigeminal nerve in the right petrosal portion of the temporal bone, where it was cut away from the portion of the neoplasm that compressed the brainstem. Note the normal maxillary nerve on the dorsal surface of the left medial pterygoid muscle on the floor of the left orbit. Compare this with the floor of the right orbit, where the denervated muscle is severely atrophied and the maxillary nerve is enlarged by the nerve sheath neoplasm.
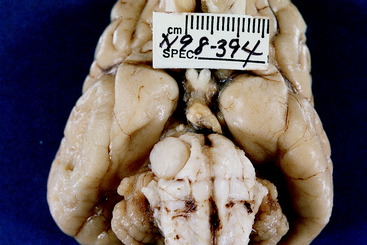
Figure 6-12 Ventral view of the brain of the dog in Figs. 6-10 and 6-11. Note the continuation of the nerve sheath neoplasm in the right trigeminal nerve at its attachment to the pons.
Lymphoma
Lymphoma can also develop within this cranial nerve, especially in cats.25 Video 6-1 shows Smokey, a 10-year-old spayed female domestic shorthair cat, who had been unable to close her mouth for 10 days during which time she was fed and watered by hand. In addition to the dropped lower jaw that she could not move, there was bilateral facial analgesia due to the loss of function of the GSA neurons in all three branches of the trigeminal nerves. Note the spontaneous blinking of the eyelids but no palpebral reflex because of the loss of the trigeminal sensory neurons. Also note the normal menace response and the ear movement, which supports normal functioning of the facial neurons. There is normal nociception in the ear because it has numerous sources of sensory innervation in addition to the small area supplied by the trigeminal nerve. Figures 6-15 through 6-17 show the brain of this cat and the bilaterally enlarged trigeminal nerves caused by an infiltrating lymphoma.
Trigeminal Neuritis
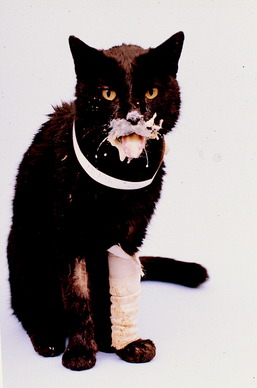
The most common cause of sudden inability to close the mouth in dogs is a bilateral nonsuppurative trigeminal neuritis.23 This is also referred to as trigeminal neuropathy, but it is an inflammatory disease that is thought to be an autoimmune disorder. In my (Alexander de Lahunta) necropsy study of one affected dog, this inflammatory lesion was in all components of the trigeminal nerve bilaterally, including the ganglia. Demyelination was prominent and axonal degeneration was rare, indicating that this was predominantly a primary demyelination. There were numerous lymphoplasmatic perivascular cuffs and many macrophages engulfing the degenerate myelin. This lesion is similar to that found in the ventral roots of dogs with polyradiculoneuritis. When you examine dogs with trigeminal neuritis, you find that the lower jaw has dropped and just hangs loose. You can manually close the mouth by elevating the lower jaw, but when you release it, the lower jaw drops back to its initial position. Usually, these dogs have normal use of their tongues and can swallow when food or water is placed in the caudal portion of the oral cavity and oral pharynx. Occasionally, swallowing may be difficult for a few days. Saliva accumulates around the lips and nose (Fig. 6-18). About one third of these dogs also has loss of facial sensation due to the involvement of the GSA neurons that are abundant in all three major branches of this nerve. A few dogs exhibit sympathetic paralysis of the head, which is referred to as Horner syndrome. This is presumed to be due to the involvement of the postganglionic sympathetic neurons that join the ophthalmic nerve from the trigeminal nerve to reach the smooth muscle of the eye, eyelids, and periorbita (Fig. 6-19). Rarely there is mild facial neuritis and paresis. Most of these dogs spontaneously recover in about 3 weeks, with or without significant atrophy of the masticatory muscles. The degree of atrophy reflects the degree of axonal involvement. Unilateral cases may be overlooked because they will be recognized only by this atrophy. MR imaging shows diffuse enlargement of the affected nerves, with hyperintensity on T2-weighted images and contrast enhancement of T1-weighted images.31 Bilaterally affected dogs must be fed and supplied with water by hand for the first 7 to 10 days. We do not treat these dogs with immunosuppressive drugs such as corticosteroids because there is no evidence that they have any influence on the rate of recovery. That probably reflects the degree of axonal injury. Recovery is short if the axons have been spared and only remyelination is necessary. Recurrences of this disorder are uncommon. We have made this clinical diagnosis in cats, but it is much less common in that species (Fig. 6-20). Despite what has been written, you cannot injure the mandibular nerve branches of the trigeminal nerve that innervate these muscles by opening the mouth too wide. The following two videos are examples of this disorder.
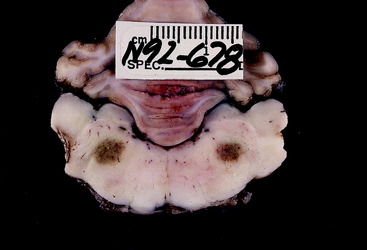
Encephalitis that involves the motor nuclei of the trigeminal nerve bilaterally causes paresis or paralysis of the muscles of mastication that results in a paretic or paralyzed dropped jaw and an open mouth that cannot be closed to prehend food. Infections by Listeria monocytogenes in ruminants and by Sarcocystis neurona in horses are examples of this (Figs. 6-21 through 6-23).
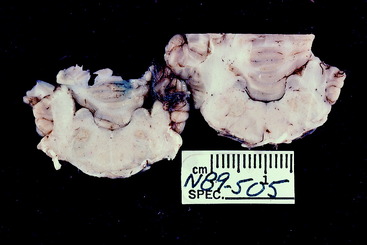
Figure 6-22 Transverse section of the pons and rostral medulla of the goat in Fig. 6-21. Note the slight bilateral discoloration in the pons, where the necrosis affected both of the motor nuclei of the trigeminal nerves.
Myositis
Myositis of the muscles of mastication is described in Chapter 5. It is not a cause of dropped lower jaw and the inability to close the mouth and has no relationship to any dysfunction of the trigeminal nerve. In the acute stage, when the muscles are swollen, any movement of the jaw causes extreme discomfort. In the chronic stage, when the muscles are severely atrophied, the fibrosis that has developed in the muscles may mechanically prevent the jaw from being opened. This myositis is treated by long-term corticosteroids and more recently by oral cyclosporine.
CRANIAL NERVE VII: FACIAL NEURONS
Anatomy
The cell bodies of the GSE neurons in the facial nerve are located in the facial nucleus in the rostral medulla. This well-defined nucleus is ventrolateral in the medulla, midway between the pyramid medially and the spinal nucleus of the trigeminal nerve laterally, caudal to the trapezoid body and the level of attachment of the caudal cerebellar peduncle to the cerebellum (see Fig. 2-13). It is caudal to the dorsal nucleus of the trapezoid body and rostral to the olivary nucleus (see Fig. 6-24; see also Figs. 6-1 and 6-3).
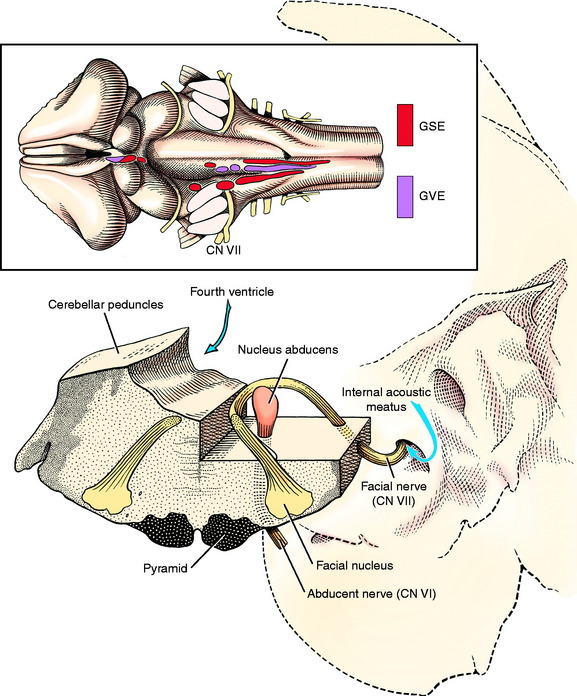
Figure 6-24 Transverse section of the pons at the level of the motor nucleus of the trigeminal nerve.
The axons of these cell bodies initially pass dorsomedially to the midline of the floor of the fourth ventricle. Here they course rostrally for 1 to 2 mm, dorsal to the abducent nucleus in the genu of the facial nerve. Then they course ventrolaterally through the medulla, medial to the spinal nucleus and tract of the trigeminal nerve and lateral to the dorsal nucleus of the trapezoid body. They emerge through the lateral aspect of the trapezoid body ventral to the vestibulocochlear nerve (see Fig. 2-12). The facial nerve accompanies the vestibulocochlear nerve into the internal acoustic meatus of the petrosal portion of the temporal bone.1 The facial nerve is more dorsal; it enters the facial canal within the temporal bone and emerges through the stylomastoid foramen. Branches of the facial nerve are distributed to the muscles of facial expression. These are the relatively thin muscles of the ear, eyelids, nose, cheeks, and lips. The facial nerve also innervates the caudal portion of the digastricus muscle, which belongs to the muscles of mastication.
Clinical Signs
Lesions of the facial nucleus or the facial nerve to the point where it emerges from the stylomastoid foramen result in complete facial paresis or paralysis and inability to move these facial muscles normally. Paralysis may be recognized by asymmetry of the face, but that depends on the species. The most obvious asymmetry occurs in the horse (Fig. 6-25); on the paralyzed side the ear droops, the palpebral fissure is decreased in size, the lower lip droops, and the nose and upper lip are markedly deviated toward the normal side because of the unopposed contractions of the muscles of the lip and nose on that side. Even the angle of the upper eyelid lashes are decreased on the affected side when the two sides are carefully compared. The ear droop, small palpebral fissure, deviated nose, and lip droop are usually seen in small ruminants, except for the ear in breeds with pendular ears like the Nubian goat. The same clinical signs are seen in cattle, but there is no deviation of the planum nasolabiale (Figs. 6-26 and 6-27). In small animals, there is no deviation of the nose and usually no decrease in size of the palpebral fissure (Fig. 6-28). The latter suggests that the levator anguli oculi medialis plays a significant role in eyelid elevation in horses and farm animals. Occasionally, this fissure is slightly enlarged on the affected side in small animals. Be aware that the sympathetic and facial nerves can be affected by middle ear disorders, and a smaller palpebral fissure may be caused by loss of the sympathetic innervation to the eye. However, this is accompanied by pupillary miosis and an elevated third eyelid. With facial paresis or paralysis, there is no ear droop in cats and in many dogs with erect ears because the ear cartilage is taut enough to keep the ear erect despite the paralysis of the ear muscles (see Figs. 6-28 and 6-35).

Figure 6-25 A 6-year-old standardbred gelding with complete right facial paralysis associated with otitis media.
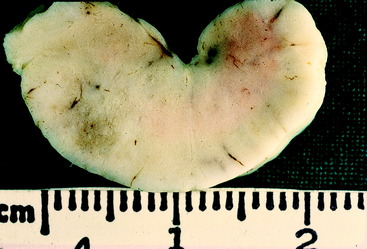
Figure 6-27 The rostral surface of a transverse section of the medulla of the cow in Fig. 6-26. Note the discoloration caused by the inflammation and necrosis at the site of the right facial nucleus (left side of photo).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree